We value our partnerships with the media and welcome any inquiries about the ACG Group. Please use the resources on this page, or if you have any specific queries please reach out to the media contact listed below.

ACG to invest $200 million in U.S. hard-shell capsule manufacturing
Dedicated facilities for gelatin and HPMC; 200+ jobs; operations targeted for early 2027; expands ACG’s 25-year U.S. presenceACG, the world’s most integrated provider of solid-dosage solutions, today announced a $200 million phased investment to establish its first empty-capsule manufacturing operations in the United States. The initial $100 million will fund a state-of-the-art hard-shell capsule facility in Atlanta, Georgia, with a second phase of $100 million planned to expand capacity and capabilities in the region. The investment is expected to create more than 200 jobs, with operations targeted to begin in early 2027.“ACG has served North America for over 25 years. This facility strengthens our ties with customers across the region—bringing us closer to them, enabling faster lead times, higher-quality service, and a more resilient, de-risked supply chain. Just as importantly, it lets us respond more quickly and co-develop new innovations through tighter R&D partnerships. It’s been a long time coming, and we’re glad to say it’s here,” said Karan Singh, Managing Director, ACG.ACG will establish dedicated facilities to manufacture gelatin and vegetarian (HPMC) hard-shell capsules, designed and operated to the highest global standards of quality, safety, and regulatory compliance. The program advances ACG’s Make it Better commitment and complements the company’s U.S. ecosystem spanning capsules, barrier packaging materials, processing and encapsulation machinery, visual inspection, and traceability solutions—supported by nationwide sales and service.“Atlanta is the right location to execute at scale,” said Selwyn Noronha, CEO, ACG Capsules. “Georgia and the City of Atlanta offer a pro-business environment, a strong talent pipeline, world-class connectivity, and reliable infrastructure. Combined with ACG’s recent facility builds in Aurangabad (HPMC), Thailand (gelatin), and expansions in Brazil, Croatia and India, we have the operating discipline to begin production quickly and deliver reliable supply for North American customers.”ACG has operated in the United States for more than 25 years, with North American headquarters in Piscataway, N.J., manufacturing liquid fill capsules in Chadds Ford, Pa., with several warehouses across the United States, and sales and service teams nationwide.About ACGFor 65 years, ACG has been innovating production solutions for pharmaceutical and nutraceutical companies that help make people better. As the world’s most integrated provider of oral-dosage products and services, we produce capsules, barrier packaging materials, manufacturing machinery, and visual inspection and traceability solutions, all fully compliant with international standards. Today, ACG partners with customers in 138 countries across six continents. Together, we share a common purpose: to solve the world’s greatest health challenges and make it better for everyone we serve.
Read more

Exclusive: ACG welcomes EC decision on titanium dioxide in pharmaceuticals
ACG, the world’s largest integrated supplier of pharmaceutical capsules and allied solutions, welcomes the European Commission’s recent announcement allowing the continued use of titanium dioxide in medicinal products.ACG has been deeply involved with this matter since the very inception of the concern, working on two fronts.On one side, the company has invested in the development of TiO2-free alternatives to support customer needs.On the other, ACG has played a key role in educating the global pharmaceutical community about the scientific possibilities and limitations in addressing the challenge.ACG is proud to have actively co-operated with the IQ Consortium, comprising 21 multinational pharmaceutical companies, in the creation of a robust database on the potential impact of a ban on TiO₂ in medicines.This scientific body of evidence played a vital role in informing the European Commission’s decision-making process.“This is a wise and pragmatic decision for the pharmaceutical sector,” said Dr Subhashis Chakraborty, General Manager, Head, Global Product Management at ACG Capsules.“And as the ongoing ban remains in place for the nutraceutical industry, we remain fully committed to supporting through innovative TiO₂-free capsule solutions.”Talking exclusively to NBR, Dr Subhashis Chakraborty (pictured) added: When the regulation on TiO₂ was first announced, it created significant uncertainty across the industry.ACG responded swiftly and responsibly, taking the time to understand the challenge in depth. Rather than taking advantage of the situation, we chose to proactively share the technical limitations and complexities associated with finding viable TiO₂-free alternatives.We made it very clear to our customers that an exact replacement for TiO₂, particularly to achieve white colour options, would not be possible with the current material science."This helped to set realistic expectations and prevent unnecessary reformulation risks."Our transparent communication enabled pharmaceutical companies, including the IQ Consortium, to make informed and strategic decisions, rather than operating in the dark.In doing so, we represented ourselves not only as market leaders but also as thought leaders, guiding the industry with expertise and insight, rather than simply responding to regulatory changes.In addition, while the regulatory changes originated in Europe, ACG also emphasised the broader global implications, especially for multinational corporations.We advised that organisations with global operations should remain aligned and proactively plan for potential ripple effects beyond the EU.As part of our commitment to supporting the industry, ACG has hosted multiple global knowledge-sharing sessions, including webinars, technical roundtables and direct consultations.These efforts continue to guide and educate the pharmaceutical community on both the regulatory landscape and the path forward for TiO₂-free innovations.
Read more

India's Basketball Revolution Begins: ACG launches India's First Professional Basketball League in association with BFI
Mumbai, June 6, 2025: Marking a transformative moment for Indian sports, ACG Sports Private Limited, has announced the launch of India’s first professional basketball league in association with the Basketball Federation of India (BFI). This groundbreaking initiative will feature structured competitive league in 5x5 and 3x3 formats for both men and women, establishing a comprehensive professional ecosystem that opens the door for athletes across the country to pursue basketball as a viable career.Designed to redefine how the sport is played, experienced, and supported in India, the league aims to shift basketball from a niche discipline into a mainstream pursuit. It will serve as a long-term catalyst for India’s presence on the global basketball stage, backed by world-class infrastructure, holistic athlete development, and robust community engagement.The league is being driven by ACG Sports Pvt. Ltd., a division of ACG, a global leader in integrated manufacturing solutions for the pharmaceutical and nutraceutical industries. Leveraging ACG’s deep-rooted commitment to societal advancement, this initiative represents a natural extension of its decade-long investment in grassroots basketball programs.To guide the league’s global strategy and operations, Mr. Jeremy Loeliger, Former CEO & Commissioner of Australia’s National Basketball League (NBL), has been appointed Director of ACG Sports. In his new role, Loeliger will lead the development of the league’s blueprint, bringing international standards of governance, competition, and commercial innovation to the Indian shores.“Basketball represents more than just a sport – it is a vehicle for building character, leadership, and opportunity," said Mr. Karan Singh, Managing Director of ACG. "Our decade-long journey, beginning with grassroots initiatives has focused on systematically making basketball accessible across India while building the professional pathways that young athletes need to thrive."He further added, "Our vision for this professional basketball league is to establish a self-sustaining ecosystem where every child with passion and talent can see a viable future in basketball in India. We're grateful for the partnership with BFI in making this dream a reality. While this is a commendable first step, we know that building a thriving basketball ecosystem will require sustained effort and collective support to reach its full potential.”ACG’s leadership role in this initiative goes far beyond league operations. As part of its long-term strategy, ACG will launch India’s first fully residential high-performance centre within the year. The academy will feature world-class facilities, attract top coaching talent from the U.S. and Australia, and offer comprehensive training programmes for players, coaches, and referees. Nutrition, mental wellness, and academic balance will be integral to the curriculum – ensuring holistic development of future stars.Mr. Aadhav Arjuna, President of the Basketball Federation of India, stated: “This partnership with ACG is unlike anything we’ve seen before. It is driven by vision, structured for long-term impact, and rooted in a clear understanding of what Indian basketball truly needs. We’re building not just visibility, but real momentum and global credibility. Our vision is to win medals at the Asian Games and Olympics”Mr. Kulvinder Singh Gill, Secretary General of the Basketball Federation of India, stated: “This league will unlock new opportunities for young Indian talent to grow, compete professionally, and gain invaluable exposure alongside international players and coaches.”The complete league framework including team structures, competition formats, player eligibility, and governance models – is currently in development with input from global basketball experts and key industry stakeholders. Detailed announcements on participation guidelines, venue requirements, and operational protocols will follow in a phased rollout.In the coming months, the newly formed League Council will begin overseeing standards and operations, while nationwide scouting initiatives, customised athlete development tracks, and dynamic fan engagement programs will begin laying the foundation for a vibrant, inclusive, and commercially sustainable basketball culture in India.About Basketball Federation of India (BFI)The Basketball Federation of India (BFI) is the governing and controlling body of basketball in India. It is responsible for the development and promotion of the sports at all levels. BFI manages all the national-level basketball operations in India.First national level tournaments were organized in India in 1934. The India national basketball team became a member of FIBA in 1936. The governing body, BFI, was formed in 1950. Mr. Aadhav Arjuna is the President of Basketball Federation of India.About ACGFor over 60 plus years, ACG has been innovating the production solutions for pharmaceutical and nutraceutical companies, that help make people better.As the world’s most integrated provider of oral dosage products and services, we produce capsules, barrier packaging materials, manufacturing machinery, and visual inspection and traceability solutions. All fully compliant with international standards.Today, ACG fosters long-term collaborative partnerships with customers in 138 countries across six continents.Together, we share a common purpose: to solve the world’s greatest health challenges and make it better for everybody we serve.ACG Sports Private Limited is a wholly owned subsidiary of ACG, and while it operates in a different sector, it is firmly grounded in the same institutional principles of precision, scalability, and long-term value creation.For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

ACG Packaging Materials GHG Commitment Validated and Approved by SBTi
ACG Packaging Materials, a leading global supplier of integrated manufacturing solutions for the pharmaceutical and nutraceutical industries, has taken a significant step forward in its sustainability journey as the Science Based Targets initiative (SBTi) officially validated the company’s greenhouse gas (GHG) emission reduction targets. This validation aligns with ACG’s core philosophy of Making it Better, reinforcing its commitment to impactful progress toward a sustainable future.ACG Packaging Materials has committed to reducing absolute Scope 1 and 2 GHG emissions by 90%. The company has also committed to reducing Scope 3 GHG emissions from purchased goods and services, fuel and energy-related activities, and upstream transportation and distribution by 97% per tonne of products produced within the same timeframe.Speaking on this milestone, Karan Singh, Managing Director of ACG, said, “At ACG, we recognise that the future of the pharmaceutical and nutraceutical industries depends on our ability to innovate responsibly and operate sustainably. This validation provides us with a clear, science-backed framework to drive meaningful progress in reducing our environmental impact. As we move forward, our focus will be on integrating sustainable practices across our operations, investing in advanced technologies, and collaborating with stakeholders to build a resilient, low-carbon future. This milestone is a testament to our dedication to making it better while ensuring that our industry remains aligned with global climate goals”Mr. Shivshankar S.R, CEO of ACG Packaging Materials, “The SBTi validation solidifies our commitment to a business model that thrives in a carbon-conscious future. We're not simply aiming for incremental improvements; we're undertaking a fundamental shift in how we operate. Our ambitious interim targets—reducing absolute Scope 1 and 2 emissions by 54.6% and Scope 3 emissions by 61.07% per tonne of products by FY2033—demonstrate our dedication to tangible progress. This commitment enhances our ability to attract sustainability-focused investors, fortifies our supply chain, and establishes ACG as a leader in responsible manufacturing”Sunil Kumar, Head of CSR and Sustainability at ACG said, “This validation is a testament to our unwavering commitment to environmental stewardship and social responsibility. At ACG, we believe that business success is intrinsically linked to the well-being of our planet and communities. Our focus now is on expanding our sustainability initiatives beyond our operations, engaging our employees, and partnering with local communities to create a lasting positive impact. We are dedicated to building a more sustainable and equitable future for all”The Science Based Targets initiative (SBTi), a collaboration between the Carbon Disclosure Project (CDP), United Nations Global Compact (UNGC), World Resources Institute (WRI), and Worldwide Fund (WWF), provides a globally recognized framework for companies to set GHG reduction targets aligned with climate science. It specifies how much and how quickly emissions must be reduced to limit global warming to well below 1.5°C above pre-industrial levels.
Read more

ACG to boost Mexico presence with appointment of Jessica Alfaro
ACG Engineering, a division of ACG, the world's only integrated pharmaceutical solutions and manufacturing company, is delighted to announce the appointment of Jessica Alfaro as territory sales manager for the Mexico region, as the company seeks to vastly increase its presence in the territory. In her new role, Jessica Alfaro will be responsible for promoting ACG’s portfolio of process and packaging machinery in Mexico, establishing new bonds with customers through the commitment of providing integral solutions to improve their processes. With a passion for engineering, Jessica previously worked as a sales engineer for Nicolás, Sven, Pacheco Y Andresen, Design and Engineering. Her responsibilities included the monitoring of technical specifications and industry standards, as well as the continuous development of the overall product offering. She also led board sessions, assigning requirements to valid use cases that highlighted the capabilities of the product. In addition, she worked closely with customers and the engineering team to determine the needs of the process and the requirements of the system. Commenting on the appointment, John Carey, vice-president of Sales at ACG Engineering, said: “We’re delighted to welcome Jessica into this pivotal new role. ACG is currently placing real focus and investment in the Mexico region for our process and packing machinery supporting both pharmaceutical and nutraceutical manufacturers with state-of-the-art technologies. We believe her experience and dedication will play a key part in building strong customer relations in the area.”Jessica Alfaro added: “I am delighted to be joining ACG, and embracing the challenges associated with gaining a deep understanding of our clients and providing them with the best solutions to help drive their business success. “I am excited by the idea of applying my analytical skills to design efficient and sustainable processes. My chemical engineering background gives me the opportunity to merge my passions for problem solving and teamwork, allowing me to contribute to the development of technologies and solutions that have a positive impact on the world around us.”About ACGFor over sixty years, ACG has been innovating the production solutions for pharmaceutical and nutraceutical companies, that help make people better. As the world’s most integrated provider of oral dosage products and services, we produce capsules, barrier packaging materials, manufacturing machinery, and visual inspection and traceability solutions. All fully compliant with international standards.Today, ACG fosters long-term collaborative partnerships with customers in 138 countries across six continents. Together, we share a common purpose: to solve the world’s greatest health challenges and make it better for everybody we serve. For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

ACHEMA 2024: ACG Engineering on a mission to collectively ‘Make it Better’ World’s largest integrated supplier to the solid dosage manufacturing industry to display wide range of engineering and capsules products
At ACHEMA 2024 ACG Engineering, which provides end-to-end pharmaceutical engineering solutions,will be displaying its broadest range of products to date – underlining its commitment to ‘Making it Better’ for the industry and patients alike.The all-encompassing machines displayed will include the QUEST FB I, which is a highly versatile 'plug and play' fluid-bed unit for lab-scale feasibility studies. Also on show will be the ZRO 90T - a high-yield capsule filler. As will be the ACCURA 100FF - ACG's precision capsule checkweigher, suited for medium to large production batches with a capacity of 100,000 capsules per hour. Also on display will be the PROTAB 300 – a single rotary tablet press, which is suitable for R&D, small and medium-batch production, making scalability far easier, and the SECURECOAT TC III tablet coater, designed with operator safety and for use with highly potent active pharmaceutical ingredients (HPAPIs).Borja Guerra, vice president of international sales at ACG Engineering, said: “At ACG, we deliver efficient cutting-edge technology and a highly consistent ROI ratio for our global customer base, because we actively listen to them and their needs and take a collective approach towards ‘Making it Better’.“By aligning our shared strength and cross-divisional synergies with our Capsule and Films and Foils business units we are able to offer a whole world of different and highly targeted products and services – supporting large pharmaceutical companies and generic manufacturers with equal focus.”“We are really looking forward to ACHEMA 20024 and meeting with the industry to share knowledge and insights and to hopefully forge some new and exciting partnerships, continuing the expansion of ACG’s global footprint.”ACG will be exhibiting on stand A71 in Hall 3.1, between 10-14 June in Frankfurt.-Ends-About ACG For over 60 plus years, ACG has been innovating the production solutions for pharmaceutical and nutraceutical companies, that help make people better.As the world’s most integrated provider of oral dosage products and services, we produce capsules, barrier packaging materials, manufacturing machinery, and visual inspection and traceability solutions. All fully compliant with international standards.Today, ACG fosters long-term collaborative partnerships with customers in 138 countries across six continents.Together, we share a common purpose: to solve the world’s greatest health challenges and make it better for everybody we serve. For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

Vantage Nutrition to champion ‘better delivery’ at Vitafoods Europe 2024
At this year’s Vitafoods Europe, Vantage Nutrition (an ACG group company), will be showcasing and championing the term ‘better delivery’. With a real focus on innovation, the team will be debuting the outstanding beadlet technology, a complete product offering ultimate ingredient performance.Other ACG Capsules products on show will include ACGcaps™ H+, the world's first independently certified* ‘Clean Label' eco-friendly capsule, which is made using thermogelation technology (water and cellulose only). Alongside will be the ACGcaps™ TSafe opaque and TiO2-free clean capsules, with the enhanced version offering flexibility with pigmentation options.Aaron Quinn, head of business development at Vantage Nutrition – Europe, commented: “We know that the world’s healthiest products demand the world’s cleanest capsules performance through more advanced technologies. Through our innovations, we are committed to supporting nutraceutical brands when it comes to time consuming and costly R&D.“With the development of beadlet technology we are able to offer ultimate ingredient performance and enhanced delivery. Beadlets release ingredients over time, boost absorption and improve bioavailability. And by working with one supplier, manufacturers can ensure they have full control over all processes, with the 360 service – ensuring better results and enhanced uptake.”ACG is the world’s largest integrated supplier of pharmaceutical and nutraceutical solid dosage products and services. Along with the commitment to delivering integrated solutions and cutting-edge technology, Vantage Nutrition aims to provide the most comprehensive and advanced multiphase solutions to customers globally. The team’s focus in on turning product ideas into reality fast – helping companies enable, enhance, and differentiate nutra brands - from a full-service partnership to specific value additions.ACG will be exhibiting on stand H37, between 14-16 May in Geneva.* Certifications are applicable to certain colours and/or variants.About ACG For over 60 plus years, ACG has been innovating the production solutions for pharmaceutical and nutraceutical companies, that help make people better.As the world’s most integrated provider of oral dosage products and services, we produce capsules, barrier packaging materials, manufacturing machinery, and visual inspection and traceability solutions. All fully compliant with international standards.Today, ACG fosters long-term collaborative partnerships with customers in 138 countries across six continents.Together, we share a common purpose: to solve the world’s greatest health challenges and make it better for everybody we serve. For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

ACG Becomes the World’s First Capsule Manufacturing Factory to Join the Global Lighthouse Network Community 2023-24
ACG Capsules Pithampur, India is ACG’s 1st lighthouse to join the community Karan Singh, Managing Director and Balajikasiram Sundararajan, Chief Digital Officer attend the Global Light House Network ceremony in Davos to collect the award. ACG, the world's largest integrated supplier and service provider to pharmaceutical industry celebrated the inclusion of its capsule manufacturing facility in Pithampur, India, into the esteemed Global Lighthouse Network (GLN) by the World Economic Forum at the 54th Annual Davos Summit. The World Economic Forum’s Global Lighthouse Network has acknowledged the exemplary integration of Fourth Industrial Revolution (4IR) technologies, including artificial intelligence and big data analytics, by select factories globally. These facilities have been distinguished for their commitment to enhancing efficiency, fostering sustainable development, and simultaneously advancing their workforce’s skills and safeguarding the environment.Upon receiving the award, Mr. Karan Singh, Managing Director, said: “I am delighted to receive this recognition on behalf of my team. For me the most unforgettable part of our journey wasn't any technology or efficiency milestone, but the incredible team that made it all possible. Just ordinary people, united towards one goal, bringing about innovative collaborations to push boundaries of what is possible.” He added: “One of the stand-out features of our application was the Gen-AI integration. Something that was done in the less than two weeks. In between all the debate on what Gen-AI can do to humans it is a beautiful reminder that ‘technology is brilliant, but humans drive the change’. Let's remember that!”ACG operates across 138 countries in six continents and has positioned itself as a leader in the pharmaceutical sector by focusing on high-quality capsule production, increasing responsiveness, improving production yields, and boosting workforce efficiency. The company produces billions of capsules annually and has implemented over 25 innovative applications of 4IR technologies, including the industrial internet of things (IIoT), machine learning (ML), deep learning (DL), digital twins, extended reality, and generative AI.Selwyn Noronha, CEO, ACG Capsules, added: “We are extremely proud of our first factory lighthouse. From its inception the facility has pioneered in its field, but this latest honour recognises the excellence in adopting AI at speed and scale.“Our continued future-focused approach sets new benchmarks in quality and innovation, with the aim of ensuring maximum benefit for customers, regulators and the entire pharmaceutical ecosystem.”About Global Lighthouse Network Global Lighthouse Network is a collaborative platform bringing together forward-thinking manufacturers leading the charge in adopting Fourth Industrial Revolution technologies. Leveraging innovations like artificial intelligence, 3D-printing, and big data analytics, Lighthouses drive efficiency, competitiveness, and transformative business models at scale, fostering economic growth while championing workforce augmentation, environmental protection and providing a collaborative learning journey for all-sized manufacturers globally. The Global Lighthouse Network is a World Economic Forum initiative co-founded with McKinsey & Company and counselled by an Advisory Board of industry leaders, including Contemporary Amperex Technology (CATL), Foxconn Industrial Internet, Henkel, Johnson & Johnson, Koç Holdings, Schneider Electric, and Siemens. Factories and value chains that join the network are designated by an independent panel of experts.About ACG For over 60 plus years, ACG has been innovating the production solutions for pharmaceutical and nutraceutical companies, that help make people better. As the world’s most integrated provider of oral dosage products and services, we produce capsules, barrier packaging materials, manufacturing machinery, and visual inspection and traceability solutions. All fully compliant with international standards. Today, ACG fosters long-term collaborative partnerships with customers in 138 countries across six continents. Together, we share a common purpose: to solve the world’s greatest health challenges and make it better for everybody we serve.For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

Interphex 2024: ‘ACG focuses on lowering manufacturer’s TCO’
At this year’s Interphex, ACG Engineering, part of ACG - the world's only integrated pharmaceutical solutions and manufacturing company - will be focusing on its cost-effective approach to taking generics to market.ACG’s methodology is based on speed-to-market, production efficiency and reducing manufacturing costs.Borja Guerra, vice-president of international sales at ACG, said: “As a highly experienced global supplier of process and packaging machinery of all oral solid dosage requirements, we are attuned to local market requirements. We aim to provide a low TCO (total cost of ownership) for premium pharmaceutical equipment.“With over six decades of experience partnering in the oral solid dosage space, we have taken over 8000 generics to market, working with more than 1000 pharmaceutical manufacturers to achieve this.”ACG will be showcasing its ACCURA 100 FF. The capsule checkweigher is designed specifically for precise, automatic, and continuous weighing of each capsule – whether empty, filled or partially filled, with anything from powder to pellets, and which is format free.ACG is the world’s largest integrated supplier of pharmaceutical and nutraceutical solid dosage products and services. The company will be exhibiting on stand 3515 between 16-18 April in New York.About ACG For over 60 plus years, ACG has been innovating the production solutions for pharmaceutical and nutraceutical companies, that help make people better.As the world’s most integrated provider of oral dosage products and services, we produce capsules, barrier packaging materials, manufacturing machinery, and visual inspection and traceability solutions. All fully compliant with international standards.Today, ACG fosters long-term collaborative partnerships with customers in 138 countries across six continents.Together, we share a common purpose: to solve the world’s greatest health challenges and make it better for everybody we serve. For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

ACG awarded ‘Great Place To Work’ certification for a fourth consecutive year
ACG, the world’s largest integrated supplier and service provider to the pharmaceutical industry, is delighted to announce that for the fourth consecutive year, it has achieved the ‘Great Place To Work’ certification. This certification recognises employers who create an outstanding employee experience.Within ACG, five business units (Capsules, Corporate, Scitech – Research and Development Centre, Machinery, and Inspection), have been certified as a ‘Great Place to Work’. The comprehensive study, spanning five locations in India, encompassed approximately 3000 associates across management and plant categories. The process entailed a thorough survey based on key levers that define an organization's culture.Nikita Panchal, Group Head Talent, OD and DEI at ACG, said: “Winning this award for the fourth consecutive year fills me with pride and gratitude, recognizing the collective effort of our associates. It serves as a reminder of our commitment to excellence and the fact that institution-building is at the centre of all our actions as an organization.“ACG fosters collaboration by nurturing teamwork and effective communication. We care for our associates and the business community through support initiatives, and we remain progressive by supporting change and innovation. Our associates are encouraged to embrace our values, seize opportunities for growth and contribute their unique talents to shape a bright future together.”Sunil Jha, Group CHRO of ACG Group, added: “ACG thrives on collaboration through cross-functional teamwork, and – at all times – we prioritize the wellbeing of our associates.“Winning this award is incredibly gratifying and I am appreciative that all our associates have worked together to make this happen.”-Ends-About ACGFor over sixty years, ACG has been innovating the production solutions for pharmaceutical and nutraceutical companies, that help make people better. As the world’s most integrated provider of oral dosage products and services, we produce capsules, barrier packaging materials, manufacturing machinery, and visual inspection and traceability solutions. All fully compliant with international standards.Today, ACG fosters long-term collaborative partnerships with customers in 138 countries across six continents. Together, we share a common purpose: to solve the world’s greatest health challenges and make it better for everybody we serve. For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more
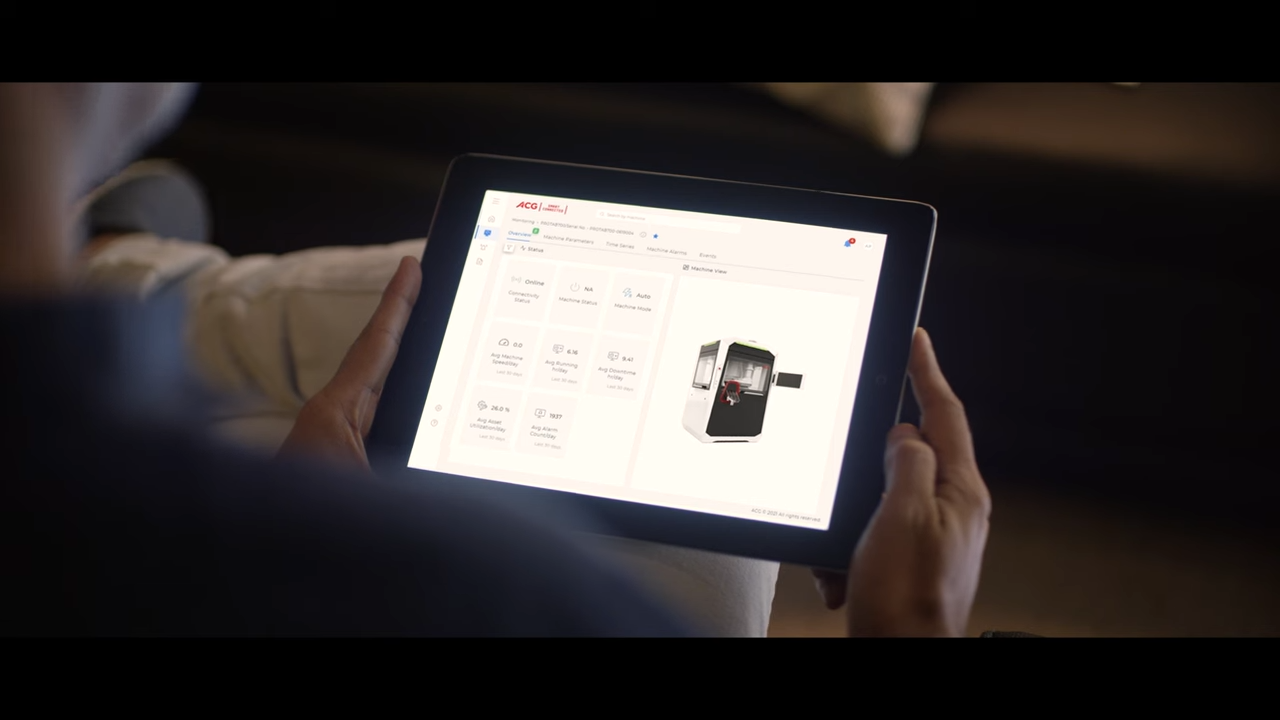
ACG Inspection launches new cloud-based offering to address upcoming VRS requirements under DSCSA regulations
In light of the impending Drug Supply Chain Security Act (DSCSA) regulations, ACG Inspection, a leading track and trace solutions provider for the pharmaceutical industry, has launched its new cloud-based Verification Router Service (VRS).The new system, which forms part of the ACG’s Inspections Life Sciences Cloud Service and Compliance Gateway, enables the automatic verification of saleable returns through product identifiers by routing requests and responses between stakeholders. Serialized products are assigned a unique identifier that can be used to track the product throughout its entire journey, enabling wholesalers to verify the authenticity of the products before they are resold.Shine Vijayan, CTO at ACG, commented: “The regulations, which have now been delayed by 12 months (coming into force in November 2024), will require all trading partners in the pharmaceutical supply chain to verify the identifier of any serialized drug product before redistributing it.“ACG’s existing VeriShield solutions tackles the implementation and interoperability challenges faced at Level 1, through to Level 3. With DSCSA’s regulation in place, ACG’s VRS covers level 4 - helping pharmaceutical manufacturers, distributors and retailers easily track and verify the saleable returns and secure their supply chain from counterfeit and substandard products.“ACG works closely with its customers, helping to address their pain points - one of which being concerns around data security. Our VRS employs robust security measures to safeguard serialized product information, guaranteeing the confidentiality and integrity of sensitive data throughout the verification process.”The system also guarantees real-time verification, to enhance operational efficiency and prevent supply chain delays. Additionally, it provides scalability assurance to accommodate an expanding volume of serialized data, to ensure continued robustness and reliability. And it incorporates exception handling, empowering stakeholders to address issues promptly to help maintain supply chain integrity.Shine Vijayan: “We are trusted experts and through our Life Sciences Cloud Service and Compliance Gateway, we can support counterfeit prevention, improving recall efficiency and data security. At all times, ensuring our clients are fully compliant with international standards and ready to meet the requirements as laid out in the impending DSCSA regulations.” -Ends- About ACGFor over sixty years, ACG has been innovating the production solutions for pharmaceutical and nutraceutical companies, that help make people better. As the world’s most integrated provider of oral dosage products and services, we produce capsules, barrier packaging materials, manufacturing machinery, and visual inspection and traceability solutions. All fully compliant with international standards.Today, ACG fosters long-term collaborative partnerships with customers in 138 countries across six continents. Together, we share a common purpose: to solve the world’s greatest health challenges and make it better for everybody we serve. For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

ACG Engineering launches the Smart Coater X.ONE Series Company’s fastest tablet coater ensures optimised production processes
ACG Engineering, a division of ACG, a leading supplier of integrated manufacturing solutions to the global pharmaceutical and nutraceutical industries, is delighted to launch its new Smart Coater X.ONE series, the company’s fastest tablet coater to date.The Smart Coater X.ONE has been designed to make tablet coating extremely fast, with an extra emphasis on process speed, efficiency and operator ease. Its advanced baffle design ensures the quickest process times for batches from 10-100%.Features of the new coater include a unique airflow pattern for optimized drying, a fully-perforated coating drum, closed dust-free charging, integrated discharge baffles, temperature sensor, a high-performing spray-arm and a 2.0 spray nozzle which has been developed with an anti-bearding cap. It also incorporates ACG’s exclusive X•ONE command and control system, designed to facilitate compliance with cGMP standards. Richard Stedman, CEO at ACG Engineering, said: “Our Smart Coater machines are already renowned for their innovative features and operator friendly designs. Now, after a lengthy period of development, we are excited to announce the launch of the X.ONE series.“Our quality-commitment philosophy means that the new machine has been crafted to achieve maximimum efficiency and flexibility for superior tablet coating, across every process – from charging to coating, discharging to cleaning. Each and every cycle is now swifter, more streamlined and profitable.“The fast tablet coater to date is already garnering real interest, and we look forward to continuing to showcase it capabilities at the upcoming CPhI event in Barcelona at the end of the month.” (XXX – may want to adapt this sentence, to reflect true accuracy)-Ends-About ACGFor over 60 years, ACG has been innovating the production solutions for pharmaceutical and nutraceutical companies, that help make people better.As the world’s most integrated provider of oral dosage products and services, we produce capsules, barrier packaging materials, manufacturing machinery, and visual inspection and traceability solutions. All fully compliant with international standards.Today, ACG fosters long-term collaborative partnerships with customers in 138 countries across six continents. Together, we share a common purpose: to solve the world’s greatest health challenges and make it better for everybody we serve.For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

ACG acquires Technical Aluminium Foil Company
Further underpinning business growth across the Middle Eastern and African marketsACG announces full shareholding ownership of Technical Aluminium Foil Company (TAFC). This strategic move further solidifies ACG's growth trajectory across the Middle Eastern and African markets.As the world's only integrated pharma manufacturing solutions company, ACG offers a diverse range of products including capsules, films, foils, engineering equipment and inspection systems. The addition of TAFC, a prominent aluminium foil packaging company based in the UAE, strengthens ACG's position as a leading provider of comprehensive packaging solutions.TAFC boasts over a decade of experience serving the pharmaceutical and food industries with its extensive range of aluminium and specialty packaging foils renowned for their exceptional high barrier properties. The company's expertise in lacquering, lamination, printing and slitting further enhances ACG’s capabilities in meeting the diverse packaging needs of its clients.This acquisition represents ACG Group's inaugural venture in the Middle East, following recent successful acquisitions of ComboCap and AquaCap in the Americas. TAFC seamlessly aligns with ACG Films and Foils' existing business operations, enabling both entities to harness their collective strengths and expertise for accelerated growth.Shivshankar S.R., CEO at ACG Films & Foils, said: “We are excited to be making our first acquisition in the UAE. This strategic collaboration will further support our work in bringing innovative and high-quality packaging products to market, while reducing lead times and improving service levels.“By joining forces, the companies will be able to leverage their combined strengths and expertise to propel the business forward in the Middle Eastern and African markets.”-Ends-About ACGIn accordance with its commitment to making the world healthier, ACG has been delivering exceptional solutions to the global pharmaceutical and nutraceutical industry for sixty years, across six continents and in a hundred countries.Collaboration is at the core of ACG’s ethos. ACG is the world’s only integrated pharma manufacturing solutions company, with products ranging from capsules to films & foils, to engineering equipment and inspection systems – all that meet international regulatory requirements. For ACG, it’s always about finding innovative solutions to the world’s greatest health challenges, together.For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

Vantage Nutrition LLC acquires ComboCap Inc
This acquisition positions Vantage Nutrition as the only company in the world supplying Sidebysideä health products to nutritional and pharmaceutical markets, for enhanced combinations.Vantage Nutrition, an ACG group company announces full shareholding ownership of ComboCap Inc (USA) and BioCap (South Africa).ComboCap is renowned for the invention and commercialization of its Sidebyside technology, the world’s first three-piece capsule health product that contains an internal divider, enabling wet and dry ingredients to be brought to market side by side, separated-but-together. Backed by 80 international patent awards ComboCap has been supplying nutritional brand customers with unique finished product solutions out of its cutting-edge cGMP plant in NJ, USA. This acquisition marks a significant milestone for Vantage Nutrition and ACG, as it further expands its technology and customer solutions footprint in North America and around the world.ACG is the world’s largest integrated supplier of pharmaceutical and nutraceutical solid dosage products and services. Vantage Nutrition, an ACG group company, already has an excellent reputation as an innovator of liquid filled capsule solutions. ComboCap marks Vantage Nutrition’s second US investment in less than a year, and first in S.A, after Philadelphia-based ‘AquaCap’ was acquired from Nestlé S.A. With this expansion of manufacturing capabilities and patented technology, along with the commitment to delivering integrated solutions and cutting-edge technology, Vantage Nutrition aims to provide the most comprehensive and advanced multiphase solutions to customers globally.Karan Singh, Managing Director at ACG, said, "As one of world’s largest producers of empty hard-shell capsules, at ACG we have often thought, what next? Strengthening our portfolio of most comprehensive vegetarian and gelatin capsules, in every imaginable size, I am thrilled to announce the acquisition of ComboCap Inc and BioCap. We now will hold the patented design and specialized equipment used inproducing the world’s first 2-in-1 capsule product with a movable membrane, becoming the sole proprietor of this technology globally.With our partners, we will usher a new era in new combinations of dietary supplements, and even non-prescription or over-the counter (OTC) remedies as well as prescription (Rx) medicines to be delivered in a single dose. This technological breakthrough is a solution to current formulation challenges with many combination therapies, including incompatible ingredients or molecules. Capsules are arguably the safest and most reliable way to deliver medicine and we at ACG strive to make it better.”Tobie Louw, a Founder and CEO of ComboCap Inc, said: “Vantage Nutrition is the perfect partner for our business, and we are very excited to be part of the ACG family. We share a passion for innovation and the commitment to bring nutraceutical and pharmaceutical customers the best possible solutions and services. By joining forces and leveraging our collective capabilities we’ll no doubt bring Sidebysideä to nutraceutical and pharmaceutical markets the world over.”-Ends-About Vantage NutritionVantage Nutrition, part of the ACG Group, is one of the world’s largest and most respected manufacturer of hard-shell liquid-fill capsule solutions. As a strategic consultancy and manufacturing partner in the nutraceutical space, Vantage Nutrition helps clients bring high-quality and innovative products to market, fast. About ACGIn accordance with its commitment to making the world healthier, ACG has been delivering exceptional solutions to the global pharmaceutical and nutraceutical industry for sixty years, across six continents and in a hundred countries.Collaboration is at the core of ACG’s ethos. ACG is the world’s only integrated pharma manufacturing solutions company, with products ranging from capsules to films & foils, to engineering equipment and inspection systems – all that meet international regulatory requirements. For ACG, it’s always about finding innovative solutions to the world’s greatest health challenges, together.For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more
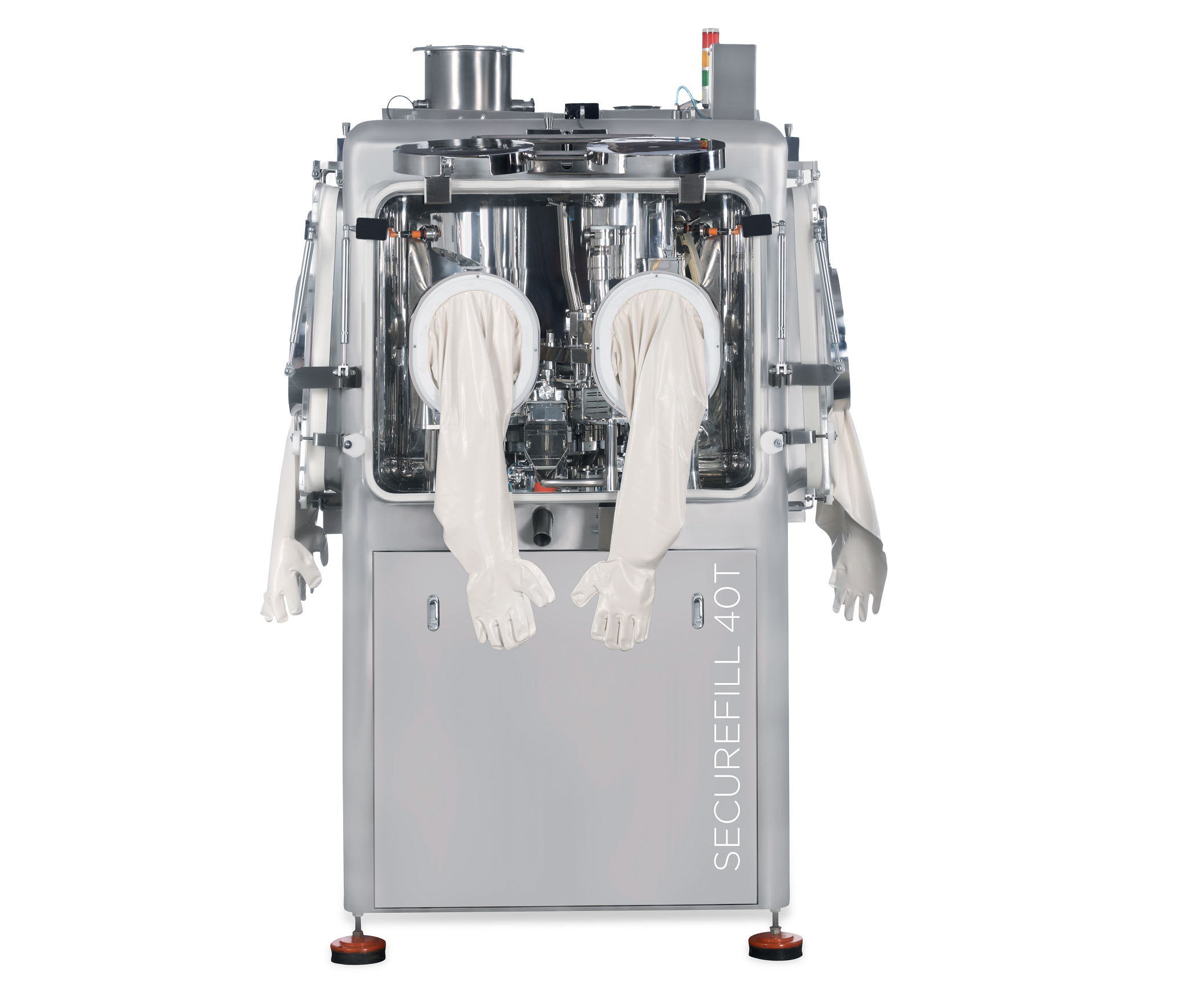
ACG Engineering – SECUREFILL 40T
Tablets & CapsulesCapsule Filling Equipment & Supplies (February 2023)ACG Engineering – SECUREFILL 40TThe Securefill series is ACG’s high-level containment capsule-filling machine range. Designed for filling capsules with highly potent and/or toxic drugs, the machines comply with occupational exposure band (OEB) up to level 4.Built with operator safety in mind, these machines are equipped for filling capsules with oncological, biopharmaceutical, antiviral formulations, and other such highly potent active pharmaceutical ingredients (HPAPIs).Features:• SS316L monoblock structure containing HEPA filters, a rapid transfer port (RTP), glove ports, and accurate dosing mechanisms maintained in a negative pressure environment.• Provision for contained charging and discharging.• Wet-in-place system to ensure wetting of all suspended particles in the pharma zone.• Can be integrated with check-weighers, metal detectors and de-dusters under containment conditions.Benefits:• Ensures complete containment, thereby avoiding operator contact within OEL range 1-10µg/m3.• Integrated containment philosophy for upstream and downstream processes.• Enables easy cleaning.• Supports the production of life-saving drugs that contain HPAPIs.Technical specifications: SECUREFILL 40T Capsule size 00 - 5 Maximum speed 40,000 capsules/hour OEL 1-10µg/m3About ACGIn accordance with its commitment to making the world healthier, ACG has been delivering exceptional solutions to the global pharmaceutical and nutraceutical industry for sixty years, across six continents and in a hundred countries.Collaboration is at the core of ACG’s ethos. ACG is the world’s only integrated pharma manufacturing solutions company, with products ranging from capsules to films & foils, to engineering equipment and inspection systems – all that meet international regulatory requirements. For ACG, it’s always about finding innovative solutions to the world’s greatest health challenges, together.For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

Vantage Nutrition LLC acquires Aquacaps from Nestlé Health Science
Vantage Nutrition’s innovative nutraceutical offering expands into North America.. Vantage Nutrition, an ACG Group company, announced on 1st December 2022 that it has acquired Philadelphia-based ‘Aquacaps’ – an asset of Nestlé Health Science. Aquacaps is a leading contract manufacturer of liquid-filled capsules within the nutritional supplement industry in the United States. Its novel liquid delivery technology allows for the liquid filling of hard gelatin and vegetarian capsules. ACG is the world’s largest integrated supplier of pharmaceutical and nutraceutical products and services. Vantage Nutrition, an ACG group company, already has an excellent reputation as an innovator of two-piece liquid fill capsule solutions. The company’s mission is to be the most efficient partner in delivering innovative and high-quality nutraceutical products to customers globally. Karan Singh, Managing Director of ACG, said: “Although ACG has been established in North America for the last 20 years, this marks our first acquisition in the territory and is a key next step in our global expansion strategy. With this increase in our footprint and manufacturing capabilities, coupled with Vantage’s innovative technologies and 360-degree service offering, we aim to provide the most advanced combination liquid-fill solutions for customers across the region.” About VantageVantage Nutrition, part of the ACG Group, is one of the world’s largest and most respected manufacturer of hard-shell liquid-fill capsule solutions. As a strategic consultancy and manufacturing partner in the nutraceutical space, Vantage helps clients bring high-quality and innovative products to market, fast. About ACGIn accordance with its commitment to making the world healthier, ACG has been delivering exceptional solutions to the global pharmaceutical and nutraceutical industry for sixty years, across six continents and in a hundred countries.Collaboration is at the core of ACG’s ethos. ACG is the world’s only integrated pharma manufacturing solutions company, with products ranging from capsules to films & foils, to engineering equipment and inspection systems – all that meet international regulatory requirements. For ACG, it’s always about finding innovative solutions to the world’s greatest health challenges, together.For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

ACG appoints Shankar Gupta as Chief Sales Officer Industry authority to re-join business in a newly created role
ACG, the world’s largest integrated pharmaceutical and nutraceutical manufacturing solutions provider, is delighted to announce that Shankar Gupta will be re-joining the business in the newly created role of Chief Sales Officer.In his new role, Shankar will be responsible for driving sales across all four ACG divisions (Films and Foils, Capsules, Inspection and Engineering) in order to continue building the company’s ‘One ACG’ agenda. Essentially, this will involve bringing further uniformity to ACG's customer outreach processes, creating more integrated solutions, and ultimately providing a consistent brand experience to ACG’s global customers, regardless of business division or location. He will also focus on scaling new initiatives.Shankar will report directly into the managing director, Karan Singh, who comments:"I am so pleased that Shankar Gupta has decided to rejoin the business during this exciting time. His understanding of ACG and its deliverables, and deep insight into our customers’ businesses will help us to align ourselves better to their changing needs. More importantly, he will champion and support better collaboration and partnership with customers.“Shankar will be pivotal in calibrating and aligning our business units to our goal of ‘Making it Better’, and our belief that everyone deserves access to good medicine."Shankar Gupta adds: “I am excited to use my experience in the pharmaceutical industry to augment ACG's growth by aligning the organisation to the changing dynamics of the global healthcare Industry.“It will certainly call for a huge collaboration of efforts both internally and with our customers, but will ultimately lead to a richer, deeper and more numerous partnerships.”-Ends-About ACGIn accordance with its commitment to making the world healthier, ACG has been delivering exceptional solutions to the global pharmaceutical and nutraceutical industry for sixty years, across six continents and in a hundred countries.Collaboration is at the core of ACG’s ethos. ACG is the world’s only integrated pharma manufacturing solutions company, with products ranging from capsules to films & foils, to engineering equipment and inspection systems – all that meet international regulatory requirements. For ACG, it’s always about finding innovative solutions to the world’s greatest health challenges, together.For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

ACG appoints Udit K Singh as CEO of ACG Inspection
ACG, a leading supplier of fully integrated manufacturing solutions to the global pharmaceutical and nutraceutical industry, is pleased to announce the appointment of Mr. Udit K Singh as the new chief executive officer (CEO) of its Inspection Division.Mr. Singh will be supporting the company as it continues its growth and global expansion. He will be responsible for ensuring that every dose of medication that ACG Inspection’s pharmaceutical and nutraceutical partners provide is manufactured and delivered exactly as intended. That is down to the fact that ACG’s visual inspection systems track and monitor the manufacturing lines to guarantee flawless, safe products.Karan Singh, managing director, ACG said: “ACG is focused on building a diverse, world-class team by hiring top international talent. We are delighted to welcome Udit Singh to ACG Inspections. He has had an illustrious career and brings diverse and unique capabilities, including a robust understanding of the pharmaceutical sector.“The team looks forward to tapping into his deep experience to enrich our global work towards making the world safer and healthier. With his knowledge and expertise in this field, Udit Singh is sure to take ACG Inspection to newer heights.”Mr. Singh added, “I am pleased to join ACG and will work to ensure that our advanced serialisation, aggregation and anti-counterfeiting solutions track and protect medicines all the way through the supply chain, and into the hands of those who need them.“We are investing substantially to ensure our track and trace platform performs seamlessly and complies with regulatory requirements all over the world. I look forward to playing an integral role, and contributing towards the continued development, working with a world-class team.”About ACGIn accordance with its commitment to making the world healthier, ACG has been delivering exceptional solutions to the global pharmaceutical and nutraceutical industry for almost sixty years, across six continents and in a hundred countries.Collaboration is at the core of ACG’s ethos, and ACG is the world’s only integrated pharma manufacturing solutions company, with products ranging from capsules to films & foils, to engineering equipment and inspection systems – all that meet international regulatory requirements. For ACG, it’s always about finding innovative solutions to the world’s greatest health challenges, together.For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

ACG Capsules launches campaign to promote problem-free pharmaceutical production
-One of the world’s largest producers of empty hard-shell capsules is on a mission to‘Love NOTHING’ when it comes to zero-problem productionACG, one of the world’s largest producers of empty hard-shell capsules, has launched its global pharmaceutical campaign: ‘ACG loves NOTHING’. The campaign is aimed at significantly improving pharmaceutical production through collaboration and industry-leading innovation. With a host of value-added services on offer, ACG has taken a pledge to strive for zero-problem pharmaceutical and nutraceutical manufacturing.Alex Robertson, CMO at ACG, said: “The messaging at the centre of this campaign is: ‘ACG loves NOTHING’ – we are putting every effort towards zero-problem production. That’s because when ‘nothing’ happens during pharmaceutical and nutraceutical production, everything runs reliably, and our customers can get the products they make to the people who need them. We will achieve this by providing the highest quality empty capsules and all the holistic advice and expertise required for seamless operations.”Founded 60 years ago, ACG was created on the simple principle of ‘Make it Better’. As the world’s largest integrated supplier of solid dosage pharmaceutical and nutraceutical products, and with the most comprehensive ranges of vegetarian and gelatin capsules globally, ACG has the scale and range to have a far-reaching impact on pharmaceutical production and human health.Selwyn Noronha, CEO ACG Capsules, added: “The pharmaceutical sector is regulated and highly competitive. Companies operating within it need a diverse range of quality capsules that help them stand out from the crowd, while also meeting demands for clean and natural products.“Beyond exceptional products, these companies need expertise and a lifetime of targeted support to ensure efficient manufacturing and guarantee regulatory compliance. With proficiency in all aspects of manufacturing from capsules to machines and protective barriers, for the last 60 years ACG has been the only company to offer this level of support, and it continues with this focus at the heart of operations.”So, at ACG, there really is something about NOTHING. The company is on a mission to drive towards problem free production and collaborating with its customers to collectively deliver on its mission of creating a healthier world.About ACGIn accordance with its commitment to making the world healthier, ACG has now been delivering exceptional solutions to the global pharmaceutical and nutraceutical industry for 60 years, across six continents and in 138 countries.‘Collaboration’ is at the core of ACG’s ethos, and ACG is the world’s only integrated pharma manufacturing solutions company, with products ranging from capsules to films & foils, to engineering equipment, and inspection systems – all that meet international regulatory requirements.For ACG, it’s always about finding innovative solutions to the world’s greatest health challenges, together.For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

How Automotive Lessons are Shaping Pharma Manufacturing
When Nikhil Kulkarni reflects on his journey into engineering, he traces it back to childhood days in Kolhapur, a town renowned for its manufacturing culture. Growing up surrounded by machines and engineers shaped his fascination with design, efficiency, and transformation. His early career in the automotive sector gave him not just technical expertise, but also a crash course in leadership after transforming a joint venture from start-up to scale-up instilled a taste for ambitious goals and complex challenges.Today, as Group CEO of ACG Engineering, Kulkarni has carried that mindset into a very different industry: pharmaceuticals. In this interview, we speak with Kulkarni about his vision for pharma manufacturing: intelligent, flexible, and sustainable systems powered by digital transformation and pharma 4.0.What first inspired your interest in engineering?Growing up in a family of engineers naturally inspired me to pursue a career in engineering. My childhood days in Kolhapur, India, a town well known for its engineering and manufacturing prowess, also influenced my path. I was always fascinated by machines and automobiles from a young age. That passion gradually developed into a deep interest in the mechanical engineering domain.And how did you also gain an interest in leadership roles?Early in my career, I had the opportunity to lead the transformation of an Indo-US joint venture in India from a “start-up to scale-up” automotive business. This experience required me to work across all management levels and functions, without boundaries. It was both challenging and rewarding, and it sparked my interest in business leadership. The success of that transformation in India gave me the confidence and appetite to take on more challenging leadership roles in the future.You have a background in the automotive sector – what drew you to pharma and the role with ACG Engineering?My decision to join ACG was shaped by both strategic opportunity and personal alignment. ACG Engineering is at a pivotal point, undergoing a transformative shift, and this offered an exciting leadership opportunity where I could apply my deep automotive expertise to a new and highly impactful sector. On a personal level, the pharma industry appealed to me because of the direct role it plays in improving health and wellbeing worldwide. This gave me an additional sense of purpose in making the move.Do you think there are lessons that pharma can learn from the automotive sector?There is a great opportunity for cross-pollination of ideas and processes between the two industries. The automotive sector has a strong track record in areas such as total quality management, operational excellence, and supply chain efficiency – all of which are valuable in pharma.Pharma can also benefit from adopting the automotive industry’s approach to innovation and new product development, where process excellence, speed to market, and customer centricity are paramount. The relentless focus on improving customer experience in automotive is another area that could positively influence pharma.How would you like to see pharma manufacturing solutions evolve in an ideal world?Pharma manufacturing solutions are being shaped by cutting-edge technology, digital transformation, regulatory change, global health demands, and sustainability requirements.Looking ahead, I see the future being defined by smart manufacturing, modular, flexible, AI/ML-driven systems and by pharma 4.0. Continuous manufacturing will continue to grow, particularly as personalized medicines and therapies advance, while sustainability and green manufacturing practices will increasingly become non-negotiable. Ideally, pharma manufacturing will evolve to be more agile, intelligent, and environmentally responsible.How important is sustainability when it comes to pharma manufacturing systems?All industries across the globe, including pharma, are going through a transformation journey in sustainability. Customers now expect not only sustainable end products, but also sustainable and green practices throughout the manufacturing process. At ACG Engineering, sustainability is becoming a core value proposition. Life-cycle assessments of equipment and CO₂ footprint reduction are central to our approach, and we are following a comprehensive and structured roadmap to achieve carbon neutrality by 2040. This commitment is not only about reducing impact but also about aligning with the expectations of our customers and the wider global community.What are your goals for your role at ACG Engineering?My goal is to lead the transformative journey at ACG Engineering with a relentless focus on enhancing the overall customer experience. This will be driven by new technology and innovation, world-class quality, and operational excellence. I also want to create an environment where our teams can thrive, contribute ideas, and feel part of shaping the future of pharma manufacturingTell us about a day in the life of a CEO…My day starts and ends with a focus on activities that bring value to our customers and stakeholders. That could mean engaging directly with customers, working with teams on technology development, or addressing operational matters. Every day, I learn something new and fascinating about our customers, products, processes, and people. Those daily insights help me navigate challenges and guide the business through external headwinds, while keeping us on track toward our long-term vision.
Read more

India's ACG pumps $200M into its first US empty-capsule production facility
ACG, a supplier and service provider for drug manufacturers, is pumping $200 million into what will be the India-based company’s first empty-capsule production facility in the U.S.An initial $100 million will go toward developing a hard-shell capsule site in Atlanta, with the remaining half slated to be spent on expanding capacity and capabilities in the region, the company said in an Oct. 20 press release.The project, when completed by early 2027, will be able to churn out gelatin and vegetarian hard-shell capsules and create more than 200 jobs, according to the company.This facility strengthens our ties with customers across the region—bringing us closer to them, enabling faster lead times, higher-quality service, and a more resilient, de-risked supply chain,” Karan Singh, ACG managing director, said in the release. “Just as importantly, it lets us respond more quickly and co-develop new innovations through tighter R&D partnerships.”The company has had a presence in the U.S. for more than a quarter-century, with its North American headquarters in Piscataway, New Jersey. The firm also operates a liquid-fill capsule facility in Chadds Ford, Pennsylvania, and several warehouses and sales teams across the U.S.The U.S. project comes less than a year after ACG announced it would significantly expand its operations in Asia by building a new, 1.89 million-square-foot capsule production plant in Rayong on Thailand’s east coast. The facility—bigger than 32 football fields—will be able to produce about 20 billion hard gelatin capsules annually, the company said at the time.That facility is expected to employ about 250 workers when completed.Founded in Mumbai, India, in 1961, ACG has spent more than six decades expanding its global footprint and touts itself as having a presence in just abut every aspect of solid dosage production, ranging from capsule production to protective packaging.
Read more

Titanium Dioxide in Medicines: The Road Ahead
Titanium Dioxide in Medicines: The Road Ahead
The European Commission, citing the EMA’s April 2024 analysis, will continue to allow titanium dioxide (TiO₂) in EU medicines under Regulation 2022/63 because no suitable replacements currently exist. TiO₂ is widely used for its whitening, opacifying, and protective qualities, including ensuring uniform tablet color and shielding active ingredients from light. The EMA projects that reformulating the entire portfolio would take between seven and 12 years.While maintaining TiO₂, the commission has encouraged pharmaceutical companies to continue researching viable alternatives. We speak with Dr. Subhashis Chakraborty, General Manager and Head of Global Product Management at ACG Capsules, to find out more about what the ruling means for pharmaceutical manufacturers.How do you see the EU decision affecting drug formulation strategies in the short and long term?We need to recall the situation of a few years ago, when a potential ban on titanium dioxide in pharmaceutical products was first indicated. The concern was global. Developing standalone strategies for Europe in a global market is not straightforward. TiO₂ was used in nearly 91,000 human and 1,600 veterinary medicines in the EU, not merely as a colorant, but as a critical opacifier ensuring stability, protection, and patient compliance.Faced with this, many scientists initiated TiO₂-free development projects as a contingency measure to avoid being caught unprepared if the ban came into force. However, the EC’s decision to continue allowing TiO₂ in medicines safeguards supply security and frees up critical resources that can now be better directed toward true innovation and patient needs rather than reactive reformulation.However, this episode has been a wake-up call for the entire ecosystem. Manufacturers have learned the importance of building resilience into product development and preparing for sudden regulatory shifts. Regulators, on the other hand, have recognized the profound impact that such decisions can have on supply chains, access, and patient safety.In the short term, drug formulation strategies will likely continue with TiO₂ as the preferred choice given its proven functionality. However, in the long term, the industry will continue to explore robust alternatives – not only as a risk-mitigation measure, but to align with trends toward natural excipients, patient-centric design, and global regulatory harmonization. The incident has instilled both caution and creativity, pushing the industry to think ahead and be better prepared for the future.What are the biggest scientific and technical challenges in developing capsules without TiO₂?The biggest challenges in developing TiO₂-free capsules stem from the fact that TiO₂ does far more than provide whiteness. It ensures opacity, masks formulation variability, creates a uniform appearance, and protects photosensitive APIs. Replicating all these functions without TiO₂ demands rethinking material science, and designing alternative colorant systems, alongside ensuring consistency at scale.One complex challenge has been comparisons with tablets. While opacifiers can work effectively on a flat, white tablet surface, their behavior in transparent capsule shells is vastly different. Another hurdle was identifying an equivalent excipient with multifunctional performance similar to TiO₂.We did not want to appear overconfident in our own path, so we consulted with independent experts, fellow excipient suppliers, and even experienced customers to validate that our development and product strategy were on the right track. This collaborative approach gave us the confidence to move forward without second-guessing, while ensuring that our solutions were rooted in both science and industry acceptance.Can you elaborate on the role of your collaboration with the IQ Consortium in influencing the regulatory decision, and what insights were gained?We contributed both technical data and practical experience from our internal studies, and we supplied customized empty hard capsules that enabled detailed experimental studies.Maintaining continuous technical dialogue with the IQ Consortium with regular feedback loops ensured that the database reflected not only scientific findings, but real-world manufacturing challenges. The outcome was a body of evidence that was fact-based, balanced, and logical, helping regulators understand both the indispensability of TiO₂ and the limitations of current alternatives.This collaboration highlighted two important insights: that TiO₂ remains technically indispensable in most medicines today, and that any move to alternatives must be gradual, evidence-based, and globally harmonized to avoid jeopardizing medicine supply.How are you preparing to adapt to potential future shifts in global excipient regulations?Further regulatory scrutiny on excipients is inevitable. As science advances and patient safety takes center stage, regulators will no longer treat excipients as “inactive.” Their role in quality, stability, bioavailability, and even patient acceptance is too critical to be overlooked. The TiO₂ debate was just the beginning. Other widely used excipients, particularly synthetic colorants, are already under discussion as authorities and consumers alike show a clear preference for natural and cleaner-label options.There’s no scientific or business sense in waiting for regulations to change. It makes more sense to actively and pro-actively anticipate them by engaging with pharmacopeias, regulatory agencies, and global standard-setting bodies to stay ahead of emerging expectations. More importantly, these insights should be used to guide innovation pipelines, ensuring that capsule solutions are not only compliant today, but “future-ready.” This includes developing alternatives with natural colors, while addressing challenges such as stability, consistency, and scalability.By collaborating with industry peers, scientific experts, and regulators, ourselves, as well as other stakeholders in this field, are helping drive a more evidence-based, science-led approach to excipient evaluation.
Read more

Debunking the myths of HPMC adoption
Debunking the myths of HPMC adoption
Factors ranging from inertia to fear of failure can dominate conversations on the shop floor and in boardrooms, subsequently hindering plans for innovation and growthThe adoption of HPMC capsules is a classic example of this reticence.Despite their growing use in nutraceuticals, their versatility, customisable release profiles, insusceptibility to the dangers of crosslinking and their consumer-friendly traits (vegetarian and biodegradable), there remains a reluctance in the industry to embrace HPMC.Conversations with many pharmaceutical manufacturers suggest that their preference is to stick with gelatin as the status quo … and that the true value and benefits of HPMC are yet to be understood or acted upon within the industry.To help quell these fears and encourage scientists to add HPMC capsules to their product lines, this article aims to bust some of the most common myths, misconceptions and excuses we’ve heard about HPMC — aiming to highlight the importance of HPMC in the future of capsule technology, drug delivery and, ultimately, patient care.Myth: gelatin is the best material for capsulesAlthough HPMC has been around for decades, is already in use globally as a coating for tablets and is recognised as safe by major global regulatory agencies, including the UK’s Medicines & Healthcare products Regulatory Agency (MHRA) and the US Food and Drug Administration (FDA), gelatin is still seen by most pharmaceutical manufacturers and formulation specialists as the “default” option when it comes to developing generic capsule formulations.This is largely because gelatin came first. Gelatin was well established as the safest, most versatile and only option for capsules for decades until the latter half of the 20th century.Owing to a lack of viable alternatives in the past, recognised challenges with gelatin, such as an unpredictable shelf-life because of cross-linking issues, were skirted around … despite causing quality challenges during the storage and transportation of the product.With safe and viable alternatives such as HPMC now readily available and widely used, it is important to note that inconsistent and costly workarounds — like adding enzymes to overcome challenges related to cross-linking in gelatin — are no longer necessary."Gelatin should no longer be thought of as the default or only option."Instead of spending valuable resources fighting and bending over backwards to accommodate gelatin’s limitations, R&D teams’ brainpower, time and budgets can be better spent investing in cellulose derivative materials such as HPMC to unlock new opportunities in capsule product innovation and development.Myth: the burden of regulatory requirements is too great to warrant investment in HPMCPharmaceutical generic manufacturers often express concerns about the regulatory flexibility involved in adopting different types of capsules, even those with similar dissolution behaviours to gelatin (such as HPMC).To avoid any regulatory headaches, they choose to limit their development to what has previously been done. This perspective not only overlooks the benefits that HPMC can bring but also its contribution to mitigating risk.This “guidance” provided by the regulatory bodies, however, is just a recommendation and not a mandatory requirement.Regardless of which capsule material they choose, each company must conduct their own studies, submit regulatory data and perform bioequivalence testing when introducing any generic products to market.If the product meets the bioequivalence requirements, the type of capsules will not be questioned; so, despite the implication, adopting HPMC should not cause any undue regulatory burden. In addition, after the upfront regulatory work, manufacturers generally have a more varied product portfolio with HPMC and also experience reduced market complaints and recalls. This saves both time and costs and improves their brand image and reputation. Myth: gelatin is the more cost-effective optionAlthough gelatin capsules generally have a lower price tag than HPMC alternatives, this cost difference primarily reflects current economies of scale rather than inherent value.As HPMC adoption continues to accelerate throughout the pharmaceutical industry, growing demand and increased competition are driving material costs down.More importantly, HPMC offers pricing stability that gelatin does not. Gelatin prices fluctuate significantly based on the availability of its raw materials — animal bones, skin and cartilage — which are subject to market volatility and supply chain disruptions.Beyond direct material costs, HPMC capsules deliver operational advantages that improve the overall economic equation.Their robust nature renders them "machine-friendly,” making them compatible with existing encapsulation equipment without requiring costly production line investments or upgrades.This eliminates any hidden transition costs that might otherwise undermine the business case for adoption.By evaluating the total cost of ownership, rather than simply comparing unit prices, factors such as improved stability, reduced rework owing to cross-interactions, fewer regulatory concerns and better compatibility with a broader range of formulations illustrate that HPMC capsules can offer significant long-term advantages compared with gelatin.Moreover, the cost of the empty hard capsule typically represents only a small fraction of the total product cost.As such, a marginal increase in capsule cost to achieve higher quality and reduce unforeseen quality issues will have minimal impact on the overall product pricing or profitability, except in cases of extremely cost-sensitive or highly commoditised products.For most formulations, this investment in quality pays dividends in product consistency, brand reputation and long-term efficiency.Myth: HPMC capsules are not proven in the marketUnderstandably, manufacturers want to work with materials that are reliable and well-established in the marketplace.Despite gaining popularity as early as the 1990s as a vegetarian alternative to gelatin, a misconception that HPMC lacks sufficient market validation still exists. This is simply untrue.Today, HPMC capsules are used widely throughout the nutraceutical industry, are considered to be safe and have been approved by major regulatory agencies, including the FDA and the European Medicines Agency, for pharmaceutical and nutraceutical applications.Since 2007, approximately 40 products in HPMC capsules have been approved by the FDA for different pharmaceutical products.This regulatory acceptance is complemented by extensive commercial adoption and documented performance.In addition, HPMC products — such as ACG's H+, HR, HA and HX capsules — offer formulation scientists the opportunity to modify drug release profiles, including valuable options for delayed-release applications.This versatility not only represents an alternative to gelatin but also the opportunity to safely use HPMC to address complex drug delivery challenges.Myth: pharmaceutical manufacturers must choose between HPMC and gelatin capsulesAdopting HPMC capsules does not mean eliminating or even phasing out the use of gelatin ones.It is not an either/or proposition; in fact, using gelatin alongside HPMC can act as a failsafe, thereby reducing risk and ensuring that there is no wasted time in product redevelopment while new offerings are being tested.Gelatin continues to be an established, reliable and widely used material in the capsules market and a suitable choice for formulations with compatible/moisture-insensitive ingredients.For products containing sensitive APIs or excipients that react to moisture, however, HPMC offers clear advantages and should be considered."The key is for formulation developers to critically evaluate different capsule materials in parallel for each specific product."This method prevents wasted time and resources in terms of failed stability studies or market complaints after many months or years of R&D, enabling a “first-time-right” approach to product launches.By making evidence-based selections, manufacturers can make room for both materials to ensure long-term market success while addressing diverse formulation needs.The future of HPMCFar from being risky or unproven, HPMC capsules have established a substantial track record of safety, regulatory compliance and formulation versatility in multiple market segments.We believe that the growing demand for industry advancements in areas including delayed release formulations, as well as the consumer-driven call for plant-based alternatives, will continue to bring HPMC and other alternatives to gelatin to the forefront.In short, HPMC is here to stay.
Read more

The rise of Asia’s largest capsule manufacturer: ACG
The rise of Asia’s largest capsule manufacturer: ACG
As the pharmaceutical industry undergoes a transformation from advanced drug formulations to patient-centric delivery systems, packaging has assumed new significance. It’s no longer just about containment; it’s about protection, traceability, and sustainability.At the forefront of this shift is ACG, a 65-year-old global leader headquartered in India, offering integrated solutions across capsules, barrier packaging, engineering systems, and inspection technologies.“We’re Asia’s largest capsule manufacturer and the second-largest globally,” says Dr Sheikh Akbar Ali, general manager and head of development and technology at ACG. “We also hold several patents that give us a significant edge, such as our capsule-in-capsule technology, which can simultaneously carry solids and liquids.”This technology, he notes, is increasingly relevant in the nutraceutical space, where complex combinations, such as oil and powder supplements, need to be delivered in single, convenient formats.Dr Sheikh Akbar Ali, general manager and head of development and technology at ACGScience behind ACG’s offeringsACG’s approach is defined by integration. Its capsules division is not just about producing hard-shell gelatin and plant-based capsules; it also offers lab support, regulatory compliance, machine compatibility testing, and customisation.The company’s vegetarian capsules are now fully plant-based. Dr Ali explains, “Our Aurangabad plant now exclusively produces these. Unlike gelatin capsules, which are sensitive to moisture and heat and may stick together or degrade, plant-based capsules are more stable and sustainable.”On the packaging side, ACG manufactures an extensive range of films and foils in-house. These include high- and ultra-high-barrier materials designed to protect medicines from moisture, oxygen, and light, critical factors in ensuring drug stability and potency.According to Dr Ali, ACG evaluates two primary barrier types: moisture and oxygen (gas) barriers. Moisture is particularly critical, as it can reduce a drug’s potency or cause degradation and impurities through hydrolytic reactions. Oxygen can lead to oxidation, for instance, causing oils to turn rancid.He says, “We measure barrier properties using instruments at varying temperatures and humidity levels, usually on flat films or foils. For new materials, we sometimes also test after forming the blister to assess real-world performance.”ACG’s blister packs include both thermoform (PVC-based) and cold-form (aluminium laminate) options. Thermoform blisters are made by heating and shaping the film. Cold-form blisters, on the other hand, don’t use heat. Instead, an aluminium-based laminate is pressed into shape at room temperature.Dr Ali says, “Cold form is essential for products sensitive to moisture, oxygen, and light, offering superior protection. However, they’re bulkier. For instance, a 10-blister cold-form pack is approximately 1.5 times the size of a thermoform.”In terms of sustainability, cold-form packs can be pyrolysed to recover aluminium, avoiding harmful emissions like dioxins (unlike PVC). While not easily recyclable, they’re safer for sensitive drugs. Thermoforms could be recycled if made from single polymers, but current combinations (polymer + aluminium) hinder that.ACG’s offeringsACG delivers solutions for the pharmaceutical and nutraceutical industries, with a strong focus on solid dosage forms. Its capsules division produces hard-shell capsules in both gelatin and plant-based HPMC variants, suitable for complex fillings such as powders, beads, liquids, tablets, and mini-capsules. Over 10,000 colour tones, pearlescent finishes, and anti-counterfeiting print options enable extensive customisation. Certified “clean label” vegetarian capsules are available, using natural pigments and titanium dioxide-free opacifiers. ACG also provides R&D support, including compatibility testing, formulation development, and stability studies.Packaging materials are manufactured entirely in-house and include high-barrier films and foils — PVC, PVDC, and PCTFE laminates — for moisture and oxygen protection. Specialised foils are offered for cold-forming, tropical climates, and child-resistant needs. Integrated solutions combine materials, machinery, and inspection systems, while the sustainability pipeline features recyclable, compostable, and halogen-free options.ACG’s engineering division supplies machinery for granulation, pelletisation, capsule filling, check-weighing, tablet compression and coating, blister packaging, cartoning, as well as vision inspection and track-and-trace systems for supply chain security.Operating in nearly 140 countries, ACG maintains a global manufacturing presence. “In packaging, we have facilities in India, Brazil, and Dubai,” says Dr Ali, “and recently opened one in Croatia to serve our European customers more closely. We're also planning more facilities in Europe within the next 18–24 months.”Packaging under pressureThe global pharmaceutical packaging industry is experiencing rapid growth. This growth is being fuelled by several key trends: ageing populations, the rise of chronic diseases, digitisation, and the increasing complexity of medicines.This complexity also comes with logistical and regulatory challenges. For instance, shipping gelatin capsules across varied climate zones poses risks. Dr Ali says, “The critical issue arises during shipping, especially by sea. Temperatures in containers can exceed 40 degrees Celsius, causing gelatin capsules to soften and stick.”He adds, “Once filled, capsules are usually bottled, and the risk decreases, unless stored in hot conditions. To mitigate this, we advise storing capsules in cool, dry places, away from sunlight. Prolonged heat exposure can also reduce drug potency.”While there is pressure to shift away from PVC due to environmental concerns, Dr Ali says the change is not imminent. “New materials must maintain drug efficacy, or regulators won’t allow their use.” Notably, global supply chains for medicinal-grade alternative materials are not in place yet. At a glanceThe global pharmaceutical packaging market is forecast to grow from USD 143.91-billion in 2024 to USD 397.71-billion by 2034, with a compound annual growth rate (CAGR) of 10.7%. North America currently leads the market, while the Asia-Pacific region is expected to experience the highest growth. Plastics and polymers dominate due to their flexibility and cost-efficiency. Primary packaging holds the largest market share and remains essential for tablets, capsules, and injectables.Source: Towards PackagingPersonalised medicinePharmaceutical packaging is increasingly being shaped by precision medicine, where treatment regimens are tailored to individual patients. This has implications for production runs, especially in blister packaging.“In the context of European healthcare systems, for instance, medicines are prescribed in a highly individualised manner. You might receive a blister pack where Monday’s dosage includes three different tablets or capsules, and Tuesday’s is different again. These are customised blisters with multiple products in a single cavity, depending on the day or the specific prescription,” Dr Ali says.Pharmaceutical packaging is increasingly being shaped by precision medicineLast month, ACG debuted the personalised capsule machine (PCM), a patented technology enabling on-demand manufacture of supplement capsules based on individual biomarkers, lifestyle choices, and health goals. Developed in collaboration with Art of You, PCM integrates real-time health data with automated production. This follows increasing demand for custom-made supplement solutions. Earlier this year, ACG also introduced the first fully vegan printed capsule for nutraceuticals.On the other hand, Dr Ali says, “We have customers with dedicated lines that run the same product with the same packaging materials for 365 days a year.” ACG’s engineering division is working to accommodate these requirements with systems that include capsule-filling machines, check-weighers, and vision inspection tools.TrendsTrends in the pharmaceutical packaging sector include smart packaging equipped with sensors and connectivity, as well as the growing use of biodegradable and recyclable materials. AI-powered inspection and packaging automation are becoming increasingly prevalent, alongside anti-counterfeiting technologies such as holography and RFID. There is also a shift towards custom, patient-centric blister formats to support personalised medicine.
Read more

How Smart Technology is helping
How Smart Technology Is Helping Reach Sustainability Goals in Drug Packaging
At conferences and trade shows up and down the calendar, bio/pharmaceutical companies take the opportunity to showcase new, updated, or redesigned equipment, responding to not only the latest demands of the market, but also the innovations of competitors. In this interview with Pharmaceutical Technology®, Sheikh Akbar Ali, PhD, general manager and head of Development and Technology for ACG Packaging Materials, reviews the state of equipment innovation, the emerging technologies that are shaping a new standard of efficiency, how global pressures are affecting the supply chain for raw materials, and what options are available to manufacturers who desire to make their processes more sustainable.A leap forward in accuracyPharmTech: To start, what are some general trends you are seeing in equipment? Smart tools and three-dimensional (3D) printing are two specific areas in which we have observed progress so far in 2025, and artificial intelligence (AI) continues to be a growing factor in all types of processes. What is most in demand at the current time?Akbar (ACG): One growing trend in pharmaceutical packaging is the move toward digital and laser printing technologies. Digital, or on-demand, printing is gaining popularity for enabling batch-level information to be printed just before medicines are packed. This supports traceability, eliminates errors, and increases patient safety as well as flexibility, especially in smaller or personalized medicine batches. Laser printing is also emerging, offering a high-energy, ink free alternative for durable and tamper-evident markings. ACG has been investing significantly in AI to develop smarter equipment. As an example, our vision inspection systems are capable of detecting even the slightest manufacturing defects, ensuring only flawless products are produced. The integration of AI has resulted in a quantum leap forward in defect-detection accuracy, particularly for low-contrast product and foil combinations. Also, training these systems now requires minimal human involvement, with the machine continuously learning and refining its performance through deep learning, and improving its own efficiencies incrementally.PharmTech: Many companies are taking steps to increase capacity to meet the demands of their customers. Is there a category of equipment that companies are tending toward including in their expansion plans?Akbar (ACG): Like many other sectors, pharmaceutical companies are highly attuned to cost efficiency and inventory management. With a growing emphasis on sustainability, they are now equally attentive to the carbon footprint of their products. As a result, packaging companies are focusing not just on capacity building, but on developing capabilities to produce packaging with the lowest possible environmental impact. Particular attention is being given to solvent-free adhesives and sealing lacquers. Coating and laminating lines that accommodate these solvent-free materials are becoming increasingly valuable. Energy efficiency is another major consideration. Technologies that capture waste heat from the process stack and reuse it to reduce overall energy consumption are gaining traction. And the demand for sustainable packaging materials has accelerated the need for advanced blistering lines, and systems that are faster, modular, and reconfigurable with minimal change over time. These innovations enable manufacturers to efficiently handle a variety of eco-friendly materials without compromising on productivity or quality. In addition, companies setting up new production lines or facilities are prioritizing digital and on-demand printing infrastructure. This enables greater operational flexibility and supports compliance with evolving regulatory requirements.Strengthening sustainabilityPharmTech: How have outsourcing relationships and partnerships evolved to meet the manufacturing needs of all concerned—especially given the currently tenuous geopolitical climate?Akbar (ACG): Global supply chain disruptions—driven by geopolitical tensions—are significantly affecting raw material sourcing and timely delivery of packaging materials to pharma companies. This creates bottlenecks in both production and delivery. The issue is particularly severe for sustainable materials needed to meet net-zero goals, as these materials are either scarce or unaffordable.PharmTech: What about single-use technology? Where do you foresee the next stage of innovation in that area, and how does that align with sustainability goals for the industry?Akbar (ACG): In pharmaceutical applications, reusing packaging is not feasible due to contamination risks. Therefore, single use remains the industry standard. However, significant innovation is occurring to align single-use packaging with sustainability goals. Key developments include, firstly, polyvinyl chloride (PVC)- and halogen-free alternatives in thermoforming and cold forming applications, such as thermoform blisters where the PVC is replaced with APET (amorphous polyethylene terephthalate), PP (polypropylene), or PE (polyethylene); cold form blisters where the PVC is replaced with PP, PE, or BOPET (biaxially oriented polyethylene terephthalate), lidding foil where the halogenated primers and heat seal lacquers (HSLs) are replaced with halogen-free alternatives, or universal sealing lacquer instead of specific lacquer for specific sealing layer. A second key development is in the reduction of mass, evidenced by a compact blister where the blister dimension has been reduced to accommodate the increased number of blisters per stroke of the blister line. A third development is in recyclable materials, namely thermoforming material where both the forming and lidding material are made of the same material and can be recycled in existing recycling infrastructure. And the last is in biodegradable and compostable materials—biodegradable PVC, which is certified to degrade faster than the regular PVC, and paper-based blister packs, which are made of certified compostable materials. These developments enhance recyclability, minimize material usage, and offer safer disposal options, bringing sustainability to single-use packaging without compromising either safety or functionality.What’s on the horizonPharmTech: Are there any new, imminent, or expected regulatory changes that companies and their clients should be aware of?Akbar (ACG): There is a notable gap in global regulations for alternative sustainable packaging materials. Current standards primarily cover traditional materials like PVC, which hampers the adoption of newer, eco-friendly options. This regulatory vacuum makes it difficult for pharmaceutical companies to switch to sustainable formats, despite technological readiness. Additionally, the European Union (EU)’s Carbon Border Adjustment Mechanism is a pressing concern. It imposes a tax on imported products, increasing the cost of non-EU packaging materials. This may impact pricing and competitiveness for companies like ACG that export into the EU market.
Read more

6 Pharma Product & Packaging Trends
6 Pharma Product & Packaging Trends
Trends shaping the pharmaceutical sector in 2025 are focused on increasing gel-capsule adoption, evolving global regulations, greater emphasis on sustainability, and additional factors impacting pharma packaging and shipping practices, according to one expert who shares his insights below.1. Gel capsule emergenceSoftgels, or gel capsules, are a leading dosage form for delivering liquid or semi-liquid pharmaceuticals. A recent Allied Market Research report cites their annual 5.4% CAGR growth set to reach $7.5 billion in 2031.“Encased in a soft gelatin or plant-based shell, these capsules provide an effective means of delivering active ingredients, making them a popular choice among consumers and manufacturers alike. With their ease of swallowing, improved bioavailability, and ability to mask unpleasant tastes, softgel capsules continue to gain traction across global markets,” according to the Allied report.The rise of gel-based capsules ushers important packaging considerations, according to Akbar Ali, PhD, general manager and head of development and technology at ACG, the global pharma packager and supplier with heavy investment in capsule production.Due to their shell composition, soft-gel capsules are more susceptible to moisture and oxygen exposure, which can impact the stability and potency of the contents. As a result, they require specialized packaging materials with more advanced barrier properties than those used for solid oral dosages, says Ali, a product developer and materials (including polymers) scientist.“These films use advanced polyvinylidene chloride (PVdC) coatings, such as Solvay’s Diofan Super B, to provide exceptional resistance to both moisture and oxygen. This makes them particularly suitable for pharmaceuticals that are highly sensitive to environmental factors, ensuring extended shelf life and product stability,” Ali says.2. Evolving global regulationsWhile regulations such as the Packaging and Packaging Waste Regulation (PPWR) currently have minimal impact on primary drug packaging, they significantly affect secondary and tertiary packaging.Among the goals, PPWR aims to “reduce packaging waste by 15% per person in each Member State by 2040, compared to 2018 levels. This will be achieved, for example, by cutting down on unnecessary packaging, especially single-use and overpackaged items…,” according to the European Chemicals Agency.Similarly, policies like the Carbon Border Adjustment Mechanism and plastic taxes are becoming critical compliance considerations for businesses, according to Ali.The European Union’s Carbon Border Adjustment Mechanism (CBAM) is a tool that puts “a fair price on the carbon emitted during the production of carbon-intensive goods that are entering the EU, and to encourage cleaner industrial production in non-EU countries” according to the European Commission.The CBAM reporting obligation applies if the combined nomenclature (CN) code of the packaging is given in the customs declaration and is covered by Annex I to the CBAM Regulation. The CN adds two digits to the HS (harmonized system) code, a globally standardized numerical method of classifying traded products managed by the World Customs Organization, according to Carbon Chain.3. Moving sustainability targetsSustainability is a priority across industries, and pharmaceutical companies are increasingly setting ambitious environmental targets, according to Ali: “Many are participating in greenhouse gas (GHG) disclosure programs and aligning with the SBTi (science-based targets initiative) to achieve carbon neutrality.” He adds that there is also growing demand for packaging solutions that are recyclable, halogen-free, and resource-efficient throughout the supply chain.”4. Product mix complexityPharmaceutical companies are diversifying their product portfolios with a greater number of smaller SKUs (stock-keeping units). Ali says this shift “adds complexity to packaging material selection and shipping logistics, requiring greater flexibility in packaging production and distribution.”5. Tight delivery deadlinesTo optimize working capital, companies are minimizing inventory storage times, making faster and more responsive supply chains essential. Advanced packaging solutions, like Ultrasafe films, enable more compact blister pack designs. These reduce material costs, storage needs, and transportation expenses — and ultimately improve cash flow management, according to Ali.6. Supply chain securityPharmaceutical companies are prioritizing partnerships with packaging suppliers that have geographically diverse manufacturing facilities to ensure uninterrupted supply, according to Ali. His company “has anticipated this need” in operations that include India, Brazil, Dubai, and elsewhere, “all of which are equipped with advanced capabilities to ensure supply chain resilience, even in uncertain environments.”
Read more
Ingredient Processing: The Way Forward for Nutraceutical Products
Ingredient Processing: The Way Forward for Nutraceutical Products
This shift in focus has given rise to the dynamic and rapidly growing nutraceuticals sector. Nutraceuticals are products derived from natural food sources that offer health benefits beyond basic nutrition. They include supplements, functional foods, and beverages designed to improve overall health, prevent chronic diseases, and enhance performance.As this segment continues to expand, nutraceuticals are becoming a crucial part of modern health and wellness, empowering individuals to take control of their health with targeted, natural solutions. Whether for supplements designed for immunity, heart health or cognitive function, consumers are increasingly seeking out products that align with their unique wellness goals. As such, the global nutraceutical market has seen remarkable growth, valued at $383.69 billion in 2022 and expected to surge to $868.38 billion by 2031. This growth reflects a strong compound annual growth rate of 9.50% from 2024 to 2031.Furthermore, advancements in nutraceutical formulations have led to the development of more innovative and effective products. These advanced products are designed to meet the growing demand for natural, scientifically backed solutions, which promote long-term health and vitality. Innovations in nutraceuticals have the potential to significantly impact the industry, but success hinges on manufacturers' ability to incorporate high-quality, stable and effective ingredients into products. To accomplish this, manufacturers must meticulously select ingredients and fine-tune processing techniques.The Need for Careful Ingredient SelectionOne of the fastest-growing areas within the nutraceutical market is functional foods, which are enhanced with additional nutrients or bioactive ingredients that offer more than basic nutrition. Functional foods, such as those fortified with vitamins, minerals, probiotics, prebiotics, fiber and Omega-3 fatty acids are gaining popularity with consumers seeking to improve their overall health and well-being through better nutrition.Alongside functional foods, dietary supplements are also experiencing rapid growth within the nutraceutical sector. These supplements are carefully formulated to address specific health concerns, such as boosting immune function, supporting digestion or enhancing cognitive performance. Whether in the form of capsules, powders, or liquids, these supplements provide a convenient way for consumers to fill nutritional gaps and achieve specific health goals, further driving the growth of the market.However, the success of both segments hinges on the careful selection of active ingredients and processing methods. The effectiveness and safety of these products are directly impacted by the quality of the components used and the formulation strategies applied. Manufacturers must meticulously select ingredients and fine-tune processing techniques in order to maintain the desirability and efficacy of the final product.To stay aligned with current trends, manufacturers are adopting advanced techniques such as coating, fluid bed processing, encapsulation, pelletization, spray drying and freeze-drying. This drive for innovation is closely linked to progress in nutraceutical ingredient processing technologies, which have become valuable assets for manufacturers seeking to enhance product quality and effectiveness.Transforming Ingredients with Advanced ProcessingIngredient processing is one of the promising and cost effective approaches helping to meet current market demands. It is a method of conversion of raw materials into value-added products that can be used as a starting product for development or as an end product. The primary objective of this process is to produce high-quality, stable and effective ingredients that can be incorporated into products to promote health and wellness.The process involves state-of-the-art techniques such as encapsulation, coating, granulation and mixing, which are used to strategically modify the physical and chemical properties of active ingredients. This transformation optimizes their functionality, boosting both the nutritional value and the overall benefits of nutraceutical products. The result is a higher standard of excellence and performance to help meet stringent industry demands.One of the key advantages of ingredient processing is its flexibility as a tool to customize products based on specific customer preferences and formulation needs. Whether enhancing product stability, improving bioavailability, or masking undesirable tastes or odors, processing technologies offer the ability to tailor products for various health and dietary applications. From aiding the manufacturing process to enabling product modifications, ingredient processing has become a vital tool in the development of high-quality nutraceuticals.Addressing Manufacturing ChallengesOne significant challenge in manufacturing, particularly when handling powdered ingredients, is the issue of dusting. This not only does it create a less efficient production process but it also leads to material wastage. Ingredient processing technology addresses this by transforming powders into microbeadlets or minibeadlets, improving the flowability of the material, reducing dust and minimizing wastage. This enhancement not only streamlines production but also ensures a more precise and consistent formulation.Another major challenge in the nutraceutical industry is the delivery of unstable liquid ingredients, extracts and oleoresins — many of which are botanically derived and unsuitable for direct use after extraction. Advanced ingredient processing technologies offer a practical solution by converting these unstable or incompatible liquid actives into stable solid forms, such as powders, pellets or beadlets. This transformation simplifies handling and delivery, while also enhancing the product’s effectiveness by ensuring better stability, shelf life and ease of use.Natural raw materials are often unstable and can degrade over time. Techniques like coating and encapsulation help stabilize these active compounds, extending their shelf life and preserving their potency throughout the product’s lifespan. Additionally, processes such as microencapsulation, nanoencapsulation and emulsification enhance the bioavailability of ingredients that are otherwise poorly absorbed, improving their absorption and effectiveness. Some nutraceutical ingredients also have strong tastes or odors that can negatively impact the consumer experience. Processing methods can mask these unwanted characteristics, making the final product more pleasant and user-friendly.Moreover, ingredient processing offers the added advantage of customizing formulations. Technologies such as blending and extrusion enable the creation of tailored products that meet specific formulation needs. By optimizing the delivery of active ingredients, these methods enhance the overall performance of products, ensuring that ingredients are utilized in a way that maximizes their impact.This approach is particularly valuable in the fortification process, where additional nutrients are integrated into products, for example: enriching protein powders with B vitamins or incorporating calcium into multivitamin blends, iodine in salt or iron in wheat and maize flour etc. This process significantly boosts the nutritional value of both nutraceuticals and food products, making them more effective in meeting dietary needs and improving public health. Also, techniques like beadlet formation further enhance this process by improving ingredient stability and controlling the release of active compounds. For instance, in Omega-3 supplements, beadlets can increase bioavailability, ensuring the body absorbs more of the active ingredient.The Evolving Nutraceutical LandscapeInnovations in nutraceuticals have the potential to significantly impact the industry, and ingredient processing plays a crucial role in this evolution. This sustainable and eco-friendly approach to raw material and final product handling is becoming increasingly important in the formulation of many products.Advancements in ingredient processing technologies are driving the development of innovative products that stand out in the market. Companies that invest in these technologies are better positioned to offer unique and high-quality products, attracting customers and maintaining a competitive edge. By leveraging these advanced technologies, the nutraceutical industry can produce products that not only meet specific health needs and consumer preferences but also satisfy the formulation need. Products formulated using these methods often achieve higher levels of purity, safety and efficacy, as required by regulatory bodies enhancing the overall quality and credibility of the brand.Ingredient processing is not just a functional necessity — it is a strategic asset for creating nutraceutical products that stand out in the market. By investing in advanced processing technologies, manufacturers can deliver safe, effective and tailored products that satisfy both regulatory standards and consumer expectations, thereby strengthening their brand and competitive edge. Author DetailsManali Dalvi, Lead, Formulation R&D, ACGManali Dalvi’s primary responsibilities include writing and publishing scientific research articles, developing segmented solutions and technical content as part of thought leadership programs. She is also involved in all the industry and institutional related collaborations and research activities. Dalvi received a Master’s in Pharmacy from Savitribai Phule Pune University.Jnanadeva Bhat, Ph.D., Vice President and Head, Formulation R&D, ACGJnanadeva Bhat is head of formulation R&D (Pharma & Nutra) at ACG Group. Bhat has been associated with the pharmaceutical industry for more than two and a half decades. As a product formulator, he has worked on various dosage forms that include tablets, soft gelatin and hard capsules, injectables and lyophilized formulations. At ACG, he heads the formulation R&D lab where he primarily leads new product development projects and customer interface.Surya Singh, Ph.D., Senior Manager Product Development (Nutra), ACGSurya Singh is part is a key member of Vantage Nutrition LLP, where he spearheads formulation development for the nutraceutical division. With over 15 years of experience in capsule formulation, Dr. Singh specializes in liquid-fi ll and pellet-fi ll technologies, bringing expertise in creating innovative, high-value nutraceutical products. His leadership drives the development of cutting-edge solutions that meet the evolving needs of the market, positioning his team at the forefront of industry advancements.Publication DetailsThis article appeared in Tablets and Capsules Magazine:Vol. 23, No. 1January/February 2025Pages: 13-15Source: https://www.tabletscapsules.com/3641-Technical-Articles/617376-Ingredient-Processing-The-Way-Forward-for-Nutraceutical-Products/
Read more

ACG to Showcase Sustainability Innovations at Pharmapack Europe 2025
ACG to Showcase Sustainability Innovations at Pharmapack Europe 2025
During Pharmapack Europe, ACG will be highlighting their CelluPod paper-based, recyclable, and compostable blister system for products that require low water vapor transmission rates (WVTR). The company is also featuring its packaging solutions that are free of polyvinyl chloride (PVC). These include its PVC and Halogen-Free Thermoforming Blister System for products requiring low to medium WVTR. ACG also provides an eco-friendly alternative in its Bio D PVC. The company’s Halogen-Free Cold Form Blister System also provides a high-performance sustainable solution without halogen and PVC.The company also announced its new head of Global Sales for ACG Packaging Materials, Jochen Scheil. With a background in mechanical engineering and more than 20 years of experience in large sales and service organizations in Europe, Asia Pacific, and the Middle East, Scheil previously held roles at Windmoeller & Hoelscher and Uhlmann Pac-Systeme."Joining ACG Packaging Materials at such a pivotal time is an incredible opportunity. I am thrilled to contribute to the company’s mission of driving innovative, sustainable packaging solutions globally,” said Scheil in the press release (1). With the launch of these products and our ongoing commitment to reducing emissions, ACG is setting a new standard for sustainability in the pharmaceutical industry.""We’re delighted to welcome Jochen to the team. His leadership and vast experience in pharmaceutical packaging, combined with the efforts of his team in showcasing our sustainable products and processes, will play a crucial role in advancing our European expansion,” said Sheikh Akbar Ali, general manager, Development and Technology of ACG Packaging Materials, in the release.At the Pharmapack event, ACG will also unveil its new state-of-the-art warehouse and slitting facility, which is located at its European site in Croatia.In October 2024, Pharmaceutical Technology EuropeTM sat down Anil Andrade, vice-president of Global Sales at ACG World, during CPHI Milan to discuss growth in demand in the pharma industry and efforts put forth to achieve sustainable operations. In the interview, Andrade talked about the changing perceptions of the industry and the importance of sustainability in pharmaceutical operations. During the past 10 to 15 years, ACG has seen global growth in demand, and more so in Europe, North America, and Asia. Andrade also emphasized efforts to achieve sustainability to reduce the impact on the environment (2).“Manufacturing capsules consumes a lot of water, and we have been trying to make our operations more efficient so that they consume less water, less paper, less plastic. In Croatia, as an example, we have reduced the paper consumption by almost 25 tons in a year. Same with plastic, we have reduced the consumption of plastic by 25 tons, and that has related to the benefits that we see going forward,” Andrade explained.Listen to the full interview with Andrade at https://www.pharmtech.com/view/cphi-milan-2024-growth-and-sustainability-in-the-pharma-industry.ReferencesACG. ACG to Showcase Sustainable Packaging Innovations at Pharmapack Paris 2025. Press Release. Jan. 20, 2025.Thomas, F. CPHI Milan 2024: Growth and Sustainability in the Pharma Industry. Oct. 18, 2024. https://www.pharmtech.com/view/cphi-milan-2024-growth-and-sustainability-in-the-pharma-industry Source: https://www.pharmtech.com/view/acg-to-showcase-sustainability-innovations-at-pharmapack-europe-2025
Read more
ACG covers every facet of the pharma process, all under one roof and seamlessly…
ACG covers every facet of the pharma process, all under one roof and seamlessly interconnected
ACG Inspection is launching its Life Sciences Cloud in India at the CPHI IndiaPMEC India show, which had its US launch at the Pack Expo US 2024. What has been the response to the US launch in terms of clients who are inquiring from clients or clients who have signed up?Existing clients who use our products across platforms were excited about ACG’s Life Sciences Cloud, as it represents a complete end-to end solution for their pharmaceutical supply chain needs. Our team has been busy engaging with clients in scenario-based demonstrations and discussing custom solutions to best fit their requirements. The feedback from existing clients has been overwhelmingly positive. New prospects are, naturally, evaluating it among competing options, comparing modules, and assessing the unique benefits we offer. We welcome their questions and are glad to engage in discussions that help them understand where we differ from others in the industry. After these in-depth discussions, we look forward to formalising partnerships with these new clients.What’s the upfront cost for a new customer or a new client (implementing this solution) versus an existing client? An existing client already has some parts of the solution and this life sciences cloud is the overlying layer which unites everything. Whereas a new customer might have bits and parts from different solution providers. Thus the interoperability between these different moving parts might be a concern. Also, how long does it take to train the staff to adapt to a new technology provider?I’ll address the transition first. We built this platform with flexibility and ease of integration in mind. For clients shifting from other providers, the Life Sciences Cloud is designed to integrate seamlessly, regardless of whether they currently use multiple vendors or a single provider. There’s no need for additional coding or customisation on the client’s end to fit into our platform.Regarding costs, aside from the recurring cloud subscription fees, we’ve structured the Life Sciences Cloud so that the cost of migration and integration is effectively zero. Our clients won’t bear any extra expenses for transitioning their systems, as we’ve designed the platform to support smooth interoperability with other systems on the floor.On the overall cost, we envision a flexible model that adapts to different client needs. For instance, the cost may be as low as per-serialnumber charges for regulatory reporting, or it could scale for broader uses like complete supply chain visibility, manufacturing analytics, and operator efficiency. Each client’s setup is unique, so costs are tailored according to the specific modules and integrations they require.As it’s an analytical and traceability solution, it would benefit a lot of pharma companies who are exporting. Is it compliant with the various supply chain regulations which are coming up like the Verification Router Service (VRS) in Drug Supply Chain Security Act in the US, etc?We’re deeply committed to regulatory compliance, with partnerships that keep us ahead of the curve. ACG collaborates closely with governing bodies, GS1 Global and similar regulatory bodies to ensure our systems meet international standards. We are the Gold Partner of GS1 Nigeria. These proactive engagements allow us to offer compliance solutions well before new regulations are mandated.Our participation with the Global Standard teams and the amount of deliberation that we do with them in terms of upcoming regulations, the amount of feedback we give, the amount of feedback they give (us) is way more advanced before the regulation actually hits the market before even it becomes a mandate.The cloud solution aims to deliver end-to-end supply chain traceability from raw material to end consumer, keeping the sanity of the integration and data exchange. This encompasses supporting global regulatory compliances, including the impending Drug Supply Chain Security Act (DSCSA) regulation.Many of our clients also use our systems to pilot new regulations, simulating production environments to understand and address potential challenges. Through these collaborations, we provide regulatory compliance deliverables early, allowing clients to validate their processes and prepare for real-world implementation.Who would be your client? Would it be a large pharma company? Would it be a midsize pharma company? A small one, which is scaling up? And a small company would have cost constraints. Can they approach ACG and expect a solution to help them scale up?ALSC gives global pharma companies the ability to visualise the entire manufacturing process on a very simplistic dashboard providing powerful insights enabling maximum OEE. Our modular technology caters to all types of pharma companies, from small-scale producers to major enterprises. Smaller companies can use selective modules, while larger organisations can leverage the full suite, integrating it across their entire supply chain. The system is also ideal for contract manufacturers (CMOs/CPOs), enabling smooth integration with diverse client systems and providing them with critical flexibility and cost efficiency.It has the range to cater to global pharma companies. When I say small, I’m talking about small in terms of volumes or small in terms of what they like to do with the L4 system in place. Vis-a-vis with big customers, big in terms of what they like to accomplish with L4 in terms of how many products they have, what the volume of the product is, how far along in the supply chain they want to track, how much regulatory reporting they want to do.What is ACG’s USP that you hope will draw customers to walk into your stall at PMEC India, and what are your expectations from the exhibition?We are one ACG. Our brand can be a bit of an enigma—are we a materials provider, a machinery manufacturer, or a technology provider? The answer is all of the above. As the pre-eminent integrated service provider of oral dosage, producing capsules, encapsulation, tabletting, barrier packaging materials, process manufacturing and packaging machinery, quality inspection systems and traceability solutions to the pharmacies of the world, ACG covers every facet of the pharmaceutical process, all under one roof and seamlessly interconnected. This unified ecosystem provides our clients with an unmatched return on investment, as they can avoid the fragmentation that often occurs when dealing with multiple vendors.ACG’s unique value lies in our holistic approach to the pharma industry, a journey that started 60 years ago with capsules and has since expanded to include everything from filling machines and packaging to advanced supply chain technology like the Life Sciences Cloud. At PMEC India, we’re excited to showcase new advancements in both machinery and technology. Our clients, many of whom are longstanding partners, will have the opportunity to explore the full capabilities of our Life Sciences Cloud, and we’re confident they’ll recognise its unique strengths over other solutions. Source: https://www.expresspharma.in/acg-covers-every-facet-of-the-pharma-process-all-under-one-roof-and-seamlessly-interconnected/
Read more

Q&A: ACG expands in Croatia - ACG speaks exclusively to European Pharmaceutical…
Q&A: ACG expands in Croatia - ACG speaks exclusively to European Pharmaceutical Manufacturer
1. With the expansion of capsule production capabilities at ACG's Croatian facility, how does this enhance the company's ability to meet global demand for oral solid dose capsules?The importance of increasing capacity at ACG's Croatian facilityACG's decision to expand its capsule manufacturing capacity at the Croatian facility comes at a critical time. This expansion is a consequence of our customer’s growing confidence in our supply from this plant and is designed to enhance the company's ability to meet the rising demand in Europe for high-quality empty hard capsules. By significantly increasing production capacity in Croatia, ACG is positioning itself as a reliable supplier to help mitigate supply chain disruptions and ensure a steady, high-quality supply of capsules for the pharmaceutical industry. This move not only strengthens ACG's position in the European market but is also an invitation to those customers who aspire to work with a progressive growing company that is investing and creating jobs locally in a region. ACG’s global expansion strategyRecognising that the Croatian facility alone cannot fully address the growing global need for empty hard capsules, ACG is taking proactive steps to expand its manufacturing capacity worldwide. The company is investing in facilities and production lines across multiple regions, ensuring that it can provide a reliable supply of capsules to markets around the globe. This expansion is critical to establishing a resilient, diversified supply chain that can withstand regional disruptions and provide pharmaceutical companies with the certainty they need to meet market demands.2. Is there a growing interest in advanced drug delivery systems?Multi-particulate formulations (MPFs) have garnered significant attention from product developers in recent years due to their superior benefits over traditional tablet formulations. These include improved drug release profiles, reduced risk of dose dumping, enhanced bioavailability, and flexibility in designing controlled release systems. As a result, MPFs are increasingly being adopted for advanced drug delivery systems, driving year-on-year growth in this area of pharmaceutical development.Since capsules are the ideal medium for encapsulating and delivering these multi-particulate formulations in a presentable and effective format, ACG plays a critical role in supporting the pharmaceutical industry’s transition to MPFs. The company recognises that providing the right empty hard capsules is essential to ensuring the success of these advanced drug delivery systems.To address this growing need, ACG is working closely with customers at the start of their development journey. By offering expert guidance on choosing the right capsule types, ACG helps prevent formulation failures later in the process, which could result in costly delays and wasted time. This proactive support ensures that pharmaceutical developers avoid making mistakes that could jeopardise their product’s success in the market.Furthermore, ACG is committed to creating awareness and educating its customers on the advancements in capsule manufacturing technologies, particularly the use of Hypromellose (HPMC)-based empty hard capsules as an alternative to traditional gelatine capsules. HPMC capsules offer distinct advantages, such as being vegetarian, suitable for a wider range of formulations, and offering better stability for certain active pharmaceutical ingredients (APIs). This shift towards HPMC capsules reflects the evolving demands of both regulatory requirements and consumer preferences for more versatile and sustainable options.By partnering with customers and providing them with the right tools and knowledge, ACG is not only supporting the growth of advanced drug delivery systems but also positioning itself as a key enabler for the pharmaceutical industry’s future success in this rapidly advancing field.3. How is ACG addressing the growing demand for patient-friendly dosage forms, such as easy-to-swallow capsules and personalised medicine?At ACG, our customer-centric mindset has enabled us to become an extremely flexible supplier, dedicated to meeting the evolving demands of the market, particularly in the development of patient-friendly dosage forms. We understand the importance of delivering medications in formats that are convenient, aesthetically appealing, and easy to use, which is why we continuously adapt our products to address these needs.Flexible sizes for ease of swallowingACG has proven its flexibility by offering capsules in a variety of sizes to ensure that drugs can be encapsulated in the smallest possible capsules, making them easier for patients to swallow. By offering a range of capsule sizes, ACG ensures that medications are delivered in a form that maximises patient compliance.Aesthetic and natural coloured capsulesACG is mindful of the aesthetic preferences of patients, and we have developed capsules in a variety of coloured combinations, including natural-coloured options. This is particularly important in overcoming the "pill effect"—a common issue where patients are reluctant to take medicines. Our natural-coloured capsules provide a more neutral, less intimidating appearance, enhancing patient acceptance.Easy-to-open capsules for swallowing difficultiesMany of our customers have proactively turned to regular capsules as an ideal solution for patients who have difficulty swallowing pills. These capsules can be opened, allowing patients to sprinkle the contents into food or beverages, making it more convenient for patients with swallowing difficulties to consume their medication without stress.Delayed-release capsules for specialised needsIn response to the growing demand for advanced drug delivery systems, ACG offers delayed-release capsules designed to protect sensitive ingredients like probiotics and enzymes. These capsules provide the necessary protection from the acidic environment of the stomach, ensuring that the active ingredients are delivered effectively to the areas where they are most needed in the digestive system.Unique capsule designs with anti-counterfeit featuresACG also offers innovative capsule designs such as Clinicaps and Tabsules. These capsules not only provide functional benefits but also include anti-counterfeit features, adding an extra layer of security for both patients and pharmaceutical manufacturers. These designs can be integrated into dosage forms to enhance brand protection and ensure that patients are receiving the correct medication.ConclusionBy staying true to its customer-centric philosophy, ACG has proven itself as a highly adaptable partner in addressing the growing demand for patient-friendly dosage forms. Our flexible approach allows us to meet the diverse needs of the healthcare industry, from offering capsules in various sizes and colours to providing advanced solutions like delayed-release colourscapsules and anti-counterfeit designs. This commitment to innovation ensures that we are well-positioned to help pharmaceutical companies deliver medications in formats that enhance patient compliance and overall treatment outcomes. Source: https://pharmaceuticalmanufacturer.media/pharmaceutical-industry-insights/pharmaceutical-manufacturing-insights/q-a-acg-expands-in-croatia/
Read more

ACG expands capsule production capabilities at its Croatian facility
ACG expands capsule production capabilities at its Croatian facility
ACG has expanded its European footprint with an upgrade to its Croatian facility.The extension of its existing site enhances its capsule shell manufacturing capacity, while also offering novel warehouse and slitting facilities.This will allow ACG to cater to the increasing demand amongst the pharmaceutical industry for capsule solutions, while also strengthening its European presence.It will also allow the company to streamline the region's supply chain.Since ACG acquired the capsule manufacturing site in Croatia, the compaby has invested more than €50m into the infrastructure and technology of the facility, while also focusing on process optimisation.This recent expansion — according to the company — will be completed by the end of 2024.CEO of ACG Capsules, Selwyn Noronha, commented: "The expansion of our Croatian facility marks a pivotal moment in our European strategy. By boosting production and logistics capabilities, we are not only improving lead times but also contributing to the local economy by creating new job opportunities.""This facility meets the highest global manufacturing standards, and we are replicating our sustainability initiatives here, ensuring our operations benefit both the environment and the communities we serve."SR Shivshankar, CEO at ACG Packaging Materials, added: "The opening of our new warehouse in Croatia is a crucial step in increasing our agility and better serving our European customers. With shorter lead times and enhanced flexibility, this warehouse will allow us to respond to market demands faster and more efficiently. We are excited for the facility to be fully operational by November 2024."ACG will showcase its offerings at CPHI Worldwide in Milan from October 8 to 10, at stand 6B20. Attendees are invited to join the team for an open meeting on October 9th, from 10:00 to 12:00, where refreshments will be served.Source: https://www.manufacturingchemist.com/acg-capsule-production-facility-capability-expansion-croatia
Read more

Ajit Singh
Ajit Singh earned his graduation degree from Cambridge University, UK, and post-graduation degree from Harvard Business School, USA. He currently resides in Mumbai, India, and travels widely to study pharmaceutical and allied industries.
Ajit is the Chairman of the ACG Group (formerly known as the Associated Capsules Group of Companies), which is headquartered in Mumbai. He has played a prominent role in the development of the pharmaceutical and allied industries in India and has served as an executive committee member of the Federation of Indian Chambers of Commerce & Industry (FICCI), a regional council member of the Confederation of Indian Industry (CII), Western Region, and other prominent pharmaceutical associations in India. He has also been conferred with prestigious awards for his contributions and thoughtful leadership to the pharmaceutical industry.
Ajit was a founder-member of the Young Presidents’ Organization – India Chapter and the first Indian to be invited to serve on its international board in the USA.

Jasjit Singh
Along with his brother, Ajit, Jasjit started the Associated Capsules Group in Mumbai in 1962.
Jasjit has always been interested in technology – particularly that relating to machine design – and earned a degree in Mechanical Engineering from London University. He has dozens of patents to his credit, and under his leadership the ACG Group has received numerous global awards for technology, excellence and social welfare.
Jasjit has been actively involved with starting new Forums of the Young Presidents’ Organisation. He served as the Chairman of the Mumbai Chapter, and helped found the Kolkata Chapter before becoming a founding President of the World Presidents’ Organisation. Jasjit has also received the Vijay Ratna Award for leadership in Industry, and recognition from UNIDO for his contribution to the fields of pharmaceutical technology and environmental science.
Jasjit’s other interests include self-education, reading, music, collecting rare works of arts and helping young contemporary artists, cooking, and travelling to different parts of the world – especially with his family.

Karan Singh
An organisational psychologist by education, Karan has transformed ACG from a modest Indian business into a world-renowned institution, revolutionising its vision and long-term strategy to ensure global growth. Karan has overseen ACG’s remarkable digital transformation, adopting breakthrough technologies that not only keep medicines safe, affordable, and accessible – but also vastly improve manufacturing and supply chains. Meanwhile, he has led a series of tuck-in acquisitions that ensure ACG pioneers continuously in terms of innovation and scale.
Passionate about converting ideas into growth opportunities, he supports entrepreneurial visions that solve real-world challenges. As an investor and mentor to more than 35 health-tech start-ups, guiding strategy, technology, expertise and networks, Karan seeks to consolidate the next generation of leadership – one that will secure better access and bridge availability across the global health industry.
Karan serves on the India Advisory Board for the India-Brazil Chamber of Commerce, is Co-Chair for FICCI LAC Regional Council, a key advisor to Nasscom, and an active contributor to the Global Lighthouse Network community of the World Economic Forum. Years of keen basketball-playing underpin his leadership, communication, and competitive spirit, and have brought about ACG-NBA Jump, a grassroots programme to give Indian basketballers a global platform.

Selwyn Noronha
CEO ACG CAPSULES

Selwyn Noronha

Nikhil Kulkarni
CEO ACG ENGINEERING

Nikhil Kulkarni

SR Shivshankar
CEO ACG PACKAGING MATERIALS

SR Shivshankar

Udit Singh
CEO ACG INSPECTION

Udit Singh

Sunil Jha
Chief Human Resource Officer

Sunil Jha

Werner Bongers
CEO SCITECH

Werner Bongers

Nitin Desai
Chief Commercial Officer

Nitin Desai

Parag Shah
Chief Financial Officer

Parag Shah

Shankar Gupta
Chief Sales Officer

Shankar Gupta

Balajikasiram Sundararajan
Chief Digital Officer

Balajikasiram Sundararajan

Alexander Robertson
Chief Marketing Officer

Alexander Robertson

Boilerplate
For over 60 plus years, ACG has been innovating the production solutions for pharmaceutical and nutraceutical companies, that help make people better.
As the world’s most integrated provider of oral dosage products and services, we produce capsules, barrier packaging materials, manufacturing machinery, and visual inspection and traceability solutions. All fully compliant with international standards.
Today, ACG fosters long-term collaborative partnerships with customers in 138 countries across six continents.
Together, we share a common purpose: to solve the world’s greatest health challenges and make it better for everybody we serve.