We value our partnerships with the media and welcome any inquiries about the ACG Group. Please use the resources on this page, or if you have any specific queries please reach out to the media contact listed below.

ACG to boost Mexico presence with appointment of Jessica Alfaro
ACG Engineering, a division of ACG, the world's only integrated pharmaceutical solutions and manufacturing company, is delighted to announce the appointment of Jessica Alfaro as territory sales manager for the Mexico region, as the company seeks to vastly increase its presence in the territory. In her new role, Jessica Alfaro will be responsible for promoting ACG’s portfolio of process and packaging machinery in Mexico, establishing new bonds with customers through the commitment of providing integral solutions to improve their processes. With a passion for engineering, Jessica previously worked as a sales engineer for Nicolás, Sven, Pacheco Y Andresen, Design and Engineering. Her responsibilities included the monitoring of technical specifications and industry standards, as well as the continuous development of the overall product offering. She also led board sessions, assigning requirements to valid use cases that highlighted the capabilities of the product. In addition, she worked closely with customers and the engineering team to determine the needs of the process and the requirements of the system. Commenting on the appointment, John Carey, vice-president of Sales at ACG Engineering, said: “We’re delighted to welcome Jessica into this pivotal new role. ACG is currently placing real focus and investment in the Mexico region for our process and packing machinery supporting both pharmaceutical and nutraceutical manufacturers with state-of-the-art technologies. We believe her experience and dedication will play a key part in building strong customer relations in the area.”Jessica Alfaro added: “I am delighted to be joining ACG, and embracing the challenges associated with gaining a deep understanding of our clients and providing them with the best solutions to help drive their business success. “I am excited by the idea of applying my analytical skills to design efficient and sustainable processes. My chemical engineering background gives me the opportunity to merge my passions for problem solving and teamwork, allowing me to contribute to the development of technologies and solutions that have a positive impact on the world around us.”About ACGFor over sixty years, ACG has been innovating the production solutions for pharmaceutical and nutraceutical companies, that help make people better. As the world’s most integrated provider of oral dosage products and services, we produce capsules, barrier packaging materials, manufacturing machinery, and visual inspection and traceability solutions. All fully compliant with international standards.Today, ACG fosters long-term collaborative partnerships with customers in 138 countries across six continents. Together, we share a common purpose: to solve the world’s greatest health challenges and make it better for everybody we serve. For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

ACG Becomes the World’s First Capsule Manufacturing Factory to Join the Global Lighthouse Network Community 2023-24
ACG Capsules Pithampur, India is ACG’s 1st lighthouse to join the community Karan Singh, Managing Director and Balajikasiram Sundararajan, Chief Digital Officer attend the Global Light House Network ceremony in Davos to collect the award. ACG, the world's largest integrated supplier and service provider to pharmaceutical industry celebrated the inclusion of its capsule manufacturing facility in Pithampur, India, into the esteemed Global Lighthouse Network (GLN) by the World Economic Forum at the 54th Annual Davos Summit. The World Economic Forum’s Global Lighthouse Network has acknowledged the exemplary integration of Fourth Industrial Revolution (4IR) technologies, including artificial intelligence and big data analytics, by select factories globally. These facilities have been distinguished for their commitment to enhancing efficiency, fostering sustainable development, and simultaneously advancing their workforce’s skills and safeguarding the environment.Upon receiving the award, Mr. Karan Singh, Managing Director, said: “I am delighted to receive this recognition on behalf of my team. For me the most unforgettable part of our journey wasn't any technology or efficiency milestone, but the incredible team that made it all possible. Just ordinary people, united towards one goal, bringing about innovative collaborations to push boundaries of what is possible.” He added: “One of the stand-out features of our application was the Gen-AI integration. Something that was done in the less than two weeks. In between all the debate on what Gen-AI can do to humans it is a beautiful reminder that ‘technology is brilliant, but humans drive the change’. Let's remember that!”ACG operates across 138 countries in six continents and has positioned itself as a leader in the pharmaceutical sector by focusing on high-quality capsule production, increasing responsiveness, improving production yields, and boosting workforce efficiency. The company produces billions of capsules annually and has implemented over 25 innovative applications of 4IR technologies, including the industrial internet of things (IIoT), machine learning (ML), deep learning (DL), digital twins, extended reality, and generative AI.Selwyn Noronha, CEO, ACG Capsules, added: “We are extremely proud of our first factory lighthouse. From its inception the facility has pioneered in its field, but this latest honour recognises the excellence in adopting AI at speed and scale.“Our continued future-focused approach sets new benchmarks in quality and innovation, with the aim of ensuring maximum benefit for customers, regulators and the entire pharmaceutical ecosystem.”About Global Lighthouse Network Global Lighthouse Network is a collaborative platform bringing together forward-thinking manufacturers leading the charge in adopting Fourth Industrial Revolution technologies. Leveraging innovations like artificial intelligence, 3D-printing, and big data analytics, Lighthouses drive efficiency, competitiveness, and transformative business models at scale, fostering economic growth while championing workforce augmentation, environmental protection and providing a collaborative learning journey for all-sized manufacturers globally. The Global Lighthouse Network is a World Economic Forum initiative co-founded with McKinsey & Company and counselled by an Advisory Board of industry leaders, including Contemporary Amperex Technology (CATL), Foxconn Industrial Internet, Henkel, Johnson & Johnson, Koç Holdings, Schneider Electric, and Siemens. Factories and value chains that join the network are designated by an independent panel of experts.About ACG For over 60 plus years, ACG has been innovating the production solutions for pharmaceutical and nutraceutical companies, that help make people better. As the world’s most integrated provider of oral dosage products and services, we produce capsules, barrier packaging materials, manufacturing machinery, and visual inspection and traceability solutions. All fully compliant with international standards. Today, ACG fosters long-term collaborative partnerships with customers in 138 countries across six continents. Together, we share a common purpose: to solve the world’s greatest health challenges and make it better for everybody we serve.For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

ACHEMA 2024: ACG Engineering on a mission to collectively ‘Make it Better’ World’s largest integrated supplier to the solid dosage manufacturing industry to display wide range of engineering and capsules products
At ACHEMA 2024 ACG Engineering, which provides end-to-end pharmaceutical engineering solutions,will be displaying its broadest range of products to date – underlining its commitment to ‘Making it Better’ for the industry and patients alike.The all-encompassing machines displayed will include the QUEST FB I, which is a highly versatile 'plug and play' fluid-bed unit for lab-scale feasibility studies. Also on show will be the ZRO 90T - a high-yield capsule filler. As will be the ACCURA 100FF - ACG's precision capsule checkweigher, suited for medium to large production batches with a capacity of 100,000 capsules per hour. Also on display will be the PROTAB 300 – a single rotary tablet press, which is suitable for R&D, small and medium-batch production, making scalability far easier, and the SECURECOAT TC III tablet coater, designed with operator safety and for use with highly potent active pharmaceutical ingredients (HPAPIs).Borja Guerra, vice president of international sales at ACG Engineering, said: “At ACG, we deliver efficient cutting-edge technology and a highly consistent ROI ratio for our global customer base, because we actively listen to them and their needs and take a collective approach towards ‘Making it Better’.“By aligning our shared strength and cross-divisional synergies with our Capsule and Films and Foils business units we are able to offer a whole world of different and highly targeted products and services – supporting large pharmaceutical companies and generic manufacturers with equal focus.”“We are really looking forward to ACHEMA 20024 and meeting with the industry to share knowledge and insights and to hopefully forge some new and exciting partnerships, continuing the expansion of ACG’s global footprint.”ACG will be exhibiting on stand A71 in Hall 3.1, between 10-14 June in Frankfurt.-Ends-About ACG For over 60 plus years, ACG has been innovating the production solutions for pharmaceutical and nutraceutical companies, that help make people better.As the world’s most integrated provider of oral dosage products and services, we produce capsules, barrier packaging materials, manufacturing machinery, and visual inspection and traceability solutions. All fully compliant with international standards.Today, ACG fosters long-term collaborative partnerships with customers in 138 countries across six continents.Together, we share a common purpose: to solve the world’s greatest health challenges and make it better for everybody we serve. For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

Vantage Nutrition to champion ‘better delivery’ at Vitafoods Europe 2024
At this year’s Vitafoods Europe, Vantage Nutrition (an ACG group company), will be showcasing and championing the term ‘better delivery’. With a real focus on innovation, the team will be debuting the outstanding beadlet technology, a complete product offering ultimate ingredient performance.Other ACG Capsules products on show will include ACGcaps™ H+, the world's first independently certified* ‘Clean Label' eco-friendly capsule, which is made using thermogelation technology (water and cellulose only). Alongside will be the ACGcaps™ TSafe opaque and TiO2-free clean capsules, with the enhanced version offering flexibility with pigmentation options.Aaron Quinn, head of business development at Vantage Nutrition – Europe, commented: “We know that the world’s healthiest products demand the world’s cleanest capsules performance through more advanced technologies. Through our innovations, we are committed to supporting nutraceutical brands when it comes to time consuming and costly R&D.“With the development of beadlet technology we are able to offer ultimate ingredient performance and enhanced delivery. Beadlets release ingredients over time, boost absorption and improve bioavailability. And by working with one supplier, manufacturers can ensure they have full control over all processes, with the 360 service – ensuring better results and enhanced uptake.”ACG is the world’s largest integrated supplier of pharmaceutical and nutraceutical solid dosage products and services. Along with the commitment to delivering integrated solutions and cutting-edge technology, Vantage Nutrition aims to provide the most comprehensive and advanced multiphase solutions to customers globally. The team’s focus in on turning product ideas into reality fast – helping companies enable, enhance, and differentiate nutra brands - from a full-service partnership to specific value additions.ACG will be exhibiting on stand H37, between 14-16 May in Geneva.* Certifications are applicable to certain colours and/or variants.About ACG For over 60 plus years, ACG has been innovating the production solutions for pharmaceutical and nutraceutical companies, that help make people better.As the world’s most integrated provider of oral dosage products and services, we produce capsules, barrier packaging materials, manufacturing machinery, and visual inspection and traceability solutions. All fully compliant with international standards.Today, ACG fosters long-term collaborative partnerships with customers in 138 countries across six continents.Together, we share a common purpose: to solve the world’s greatest health challenges and make it better for everybody we serve. For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

ACG awarded ‘Great Place To Work’ certification for a fourth consecutive year
ACG, the world’s largest integrated supplier and service provider to the pharmaceutical industry, is delighted to announce that for the fourth consecutive year, it has achieved the ‘Great Place To Work’ certification. This certification recognises employers who create an outstanding employee experience.Within ACG, five business units (Capsules, Corporate, Scitech – Research and Development Centre, Machinery, and Inspection), have been certified as a ‘Great Place to Work’. The comprehensive study, spanning five locations in India, encompassed approximately 3000 associates across management and plant categories. The process entailed a thorough survey based on key levers that define an organization's culture.Nikita Panchal, Group Head Talent, OD and DEI at ACG, said: “Winning this award for the fourth consecutive year fills me with pride and gratitude, recognizing the collective effort of our associates. It serves as a reminder of our commitment to excellence and the fact that institution-building is at the centre of all our actions as an organization.“ACG fosters collaboration by nurturing teamwork and effective communication. We care for our associates and the business community through support initiatives, and we remain progressive by supporting change and innovation. Our associates are encouraged to embrace our values, seize opportunities for growth and contribute their unique talents to shape a bright future together.”Sunil Jha, Group CHRO of ACG Group, added: “ACG thrives on collaboration through cross-functional teamwork, and – at all times – we prioritize the wellbeing of our associates.“Winning this award is incredibly gratifying and I am appreciative that all our associates have worked together to make this happen.”-Ends-About ACGFor over sixty years, ACG has been innovating the production solutions for pharmaceutical and nutraceutical companies, that help make people better. As the world’s most integrated provider of oral dosage products and services, we produce capsules, barrier packaging materials, manufacturing machinery, and visual inspection and traceability solutions. All fully compliant with international standards.Today, ACG fosters long-term collaborative partnerships with customers in 138 countries across six continents. Together, we share a common purpose: to solve the world’s greatest health challenges and make it better for everybody we serve. For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

Interphex 2024: ‘ACG focuses on lowering manufacturer’s TCO’
At this year’s Interphex, ACG Engineering, part of ACG - the world's only integrated pharmaceutical solutions and manufacturing company - will be focusing on its cost-effective approach to taking generics to market.ACG’s methodology is based on speed-to-market, production efficiency and reducing manufacturing costs.Borja Guerra, vice-president of international sales at ACG, said: “As a highly experienced global supplier of process and packaging machinery of all oral solid dosage requirements, we are attuned to local market requirements. We aim to provide a low TCO (total cost of ownership) for premium pharmaceutical equipment.“With over six decades of experience partnering in the oral solid dosage space, we have taken over 8000 generics to market, working with more than 1000 pharmaceutical manufacturers to achieve this.”ACG will be showcasing its ACCURA 100 FF. The capsule checkweigher is designed specifically for precise, automatic, and continuous weighing of each capsule – whether empty, filled or partially filled, with anything from powder to pellets, and which is format free.ACG is the world’s largest integrated supplier of pharmaceutical and nutraceutical solid dosage products and services. The company will be exhibiting on stand 3515 between 16-18 April in New York.About ACG For over 60 plus years, ACG has been innovating the production solutions for pharmaceutical and nutraceutical companies, that help make people better.As the world’s most integrated provider of oral dosage products and services, we produce capsules, barrier packaging materials, manufacturing machinery, and visual inspection and traceability solutions. All fully compliant with international standards.Today, ACG fosters long-term collaborative partnerships with customers in 138 countries across six continents.Together, we share a common purpose: to solve the world’s greatest health challenges and make it better for everybody we serve. For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more
ACG Inspection launches new cloud-based offering to address upcoming VRS requirements under DSCSA regulations
In light of the impending Drug Supply Chain Security Act (DSCSA) regulations, ACG Inspection, a leading track and trace solutions provider for the pharmaceutical industry, has launched its new cloud-based Verification Router Service (VRS).The new system, which forms part of the ACG’s Inspections Life Sciences Cloud Service and Compliance Gateway, enables the automatic verification of saleable returns through product identifiers by routing requests and responses between stakeholders. Serialized products are assigned a unique identifier that can be used to track the product throughout its entire journey, enabling wholesalers to verify the authenticity of the products before they are resold.Shine Vijayan, CTO at ACG, commented: “The regulations, which have now been delayed by 12 months (coming into force in November 2024), will require all trading partners in the pharmaceutical supply chain to verify the identifier of any serialized drug product before redistributing it.“ACG’s existing VeriShield solutions tackles the implementation and interoperability challenges faced at Level 1, through to Level 3. With DSCSA’s regulation in place, ACG’s VRS covers level 4 - helping pharmaceutical manufacturers, distributors and retailers easily track and verify the saleable returns and secure their supply chain from counterfeit and substandard products.“ACG works closely with its customers, helping to address their pain points - one of which being concerns around data security. Our VRS employs robust security measures to safeguard serialized product information, guaranteeing the confidentiality and integrity of sensitive data throughout the verification process.”The system also guarantees real-time verification, to enhance operational efficiency and prevent supply chain delays. Additionally, it provides scalability assurance to accommodate an expanding volume of serialized data, to ensure continued robustness and reliability. And it incorporates exception handling, empowering stakeholders to address issues promptly to help maintain supply chain integrity.Shine Vijayan: “We are trusted experts and through our Life Sciences Cloud Service and Compliance Gateway, we can support counterfeit prevention, improving recall efficiency and data security. At all times, ensuring our clients are fully compliant with international standards and ready to meet the requirements as laid out in the impending DSCSA regulations.” -Ends- About ACGFor over sixty years, ACG has been innovating the production solutions for pharmaceutical and nutraceutical companies, that help make people better. As the world’s most integrated provider of oral dosage products and services, we produce capsules, barrier packaging materials, manufacturing machinery, and visual inspection and traceability solutions. All fully compliant with international standards.Today, ACG fosters long-term collaborative partnerships with customers in 138 countries across six continents. Together, we share a common purpose: to solve the world’s greatest health challenges and make it better for everybody we serve. For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

ACG Engineering launches the Smart Coater X.ONE Series Company’s fastest tablet coater ensures optimised production processes
ACG Engineering, a division of ACG, a leading supplier of integrated manufacturing solutions to the global pharmaceutical and nutraceutical industries, is delighted to launch its new Smart Coater X.ONE series, the company’s fastest tablet coater to date.The Smart Coater X.ONE has been designed to make tablet coating extremely fast, with an extra emphasis on process speed, efficiency and operator ease. Its advanced baffle design ensures the quickest process times for batches from 10-100%.Features of the new coater include a unique airflow pattern for optimized drying, a fully-perforated coating drum, closed dust-free charging, integrated discharge baffles, temperature sensor, a high-performing spray-arm and a 2.0 spray nozzle which has been developed with an anti-bearding cap. It also incorporates ACG’s exclusive X•ONE command and control system, designed to facilitate compliance with cGMP standards. Richard Stedman, CEO at ACG Engineering, said: “Our Smart Coater machines are already renowned for their innovative features and operator friendly designs. Now, after a lengthy period of development, we are excited to announce the launch of the X.ONE series.“Our quality-commitment philosophy means that the new machine has been crafted to achieve maximimum efficiency and flexibility for superior tablet coating, across every process – from charging to coating, discharging to cleaning. Each and every cycle is now swifter, more streamlined and profitable.“The fast tablet coater to date is already garnering real interest, and we look forward to continuing to showcase it capabilities at the upcoming CPhI event in Barcelona at the end of the month.” (XXX – may want to adapt this sentence, to reflect true accuracy)-Ends-About ACGFor over 60 years, ACG has been innovating the production solutions for pharmaceutical and nutraceutical companies, that help make people better.As the world’s most integrated provider of oral dosage products and services, we produce capsules, barrier packaging materials, manufacturing machinery, and visual inspection and traceability solutions. All fully compliant with international standards.Today, ACG fosters long-term collaborative partnerships with customers in 138 countries across six continents. Together, we share a common purpose: to solve the world’s greatest health challenges and make it better for everybody we serve.For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

ACG acquires Technical Aluminium Foil Company
Further underpinning business growth across the Middle Eastern and African marketsACG announces full shareholding ownership of Technical Aluminium Foil Company (TAFC). This strategic move further solidifies ACG's growth trajectory across the Middle Eastern and African markets.As the world's only integrated pharma manufacturing solutions company, ACG offers a diverse range of products including capsules, films, foils, engineering equipment and inspection systems. The addition of TAFC, a prominent aluminium foil packaging company based in the UAE, strengthens ACG's position as a leading provider of comprehensive packaging solutions.TAFC boasts over a decade of experience serving the pharmaceutical and food industries with its extensive range of aluminium and specialty packaging foils renowned for their exceptional high barrier properties. The company's expertise in lacquering, lamination, printing and slitting further enhances ACG’s capabilities in meeting the diverse packaging needs of its clients.This acquisition represents ACG Group's inaugural venture in the Middle East, following recent successful acquisitions of ComboCap and AquaCap in the Americas. TAFC seamlessly aligns with ACG Films and Foils' existing business operations, enabling both entities to harness their collective strengths and expertise for accelerated growth.Shivshankar S.R., CEO at ACG Films & Foils, said: “We are excited to be making our first acquisition in the UAE. This strategic collaboration will further support our work in bringing innovative and high-quality packaging products to market, while reducing lead times and improving service levels.“By joining forces, the companies will be able to leverage their combined strengths and expertise to propel the business forward in the Middle Eastern and African markets.”-Ends-About ACGIn accordance with its commitment to making the world healthier, ACG has been delivering exceptional solutions to the global pharmaceutical and nutraceutical industry for sixty years, across six continents and in a hundred countries.Collaboration is at the core of ACG’s ethos. ACG is the world’s only integrated pharma manufacturing solutions company, with products ranging from capsules to films & foils, to engineering equipment and inspection systems – all that meet international regulatory requirements. For ACG, it’s always about finding innovative solutions to the world’s greatest health challenges, together.For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

Vantage Nutrition LLC acquires ComboCap Inc
This acquisition positions Vantage Nutrition as the only company in the world supplying Sidebysideä health products to nutritional and pharmaceutical markets, for enhanced combinations.Vantage Nutrition, an ACG group company announces full shareholding ownership of ComboCap Inc (USA) and BioCap (South Africa).ComboCap is renowned for the invention and commercialization of its Sidebyside technology, the world’s first three-piece capsule health product that contains an internal divider, enabling wet and dry ingredients to be brought to market side by side, separated-but-together. Backed by 80 international patent awards ComboCap has been supplying nutritional brand customers with unique finished product solutions out of its cutting-edge cGMP plant in NJ, USA. This acquisition marks a significant milestone for Vantage Nutrition and ACG, as it further expands its technology and customer solutions footprint in North America and around the world.ACG is the world’s largest integrated supplier of pharmaceutical and nutraceutical solid dosage products and services. Vantage Nutrition, an ACG group company, already has an excellent reputation as an innovator of liquid filled capsule solutions. ComboCap marks Vantage Nutrition’s second US investment in less than a year, and first in S.A, after Philadelphia-based ‘AquaCap’ was acquired from Nestlé S.A. With this expansion of manufacturing capabilities and patented technology, along with the commitment to delivering integrated solutions and cutting-edge technology, Vantage Nutrition aims to provide the most comprehensive and advanced multiphase solutions to customers globally.Karan Singh, Managing Director at ACG, said, "As one of world’s largest producers of empty hard-shell capsules, at ACG we have often thought, what next? Strengthening our portfolio of most comprehensive vegetarian and gelatin capsules, in every imaginable size, I am thrilled to announce the acquisition of ComboCap Inc and BioCap. We now will hold the patented design and specialized equipment used inproducing the world’s first 2-in-1 capsule product with a movable membrane, becoming the sole proprietor of this technology globally.With our partners, we will usher a new era in new combinations of dietary supplements, and even non-prescription or over-the counter (OTC) remedies as well as prescription (Rx) medicines to be delivered in a single dose. This technological breakthrough is a solution to current formulation challenges with many combination therapies, including incompatible ingredients or molecules. Capsules are arguably the safest and most reliable way to deliver medicine and we at ACG strive to make it better.”Tobie Louw, a Founder and CEO of ComboCap Inc, said: “Vantage Nutrition is the perfect partner for our business, and we are very excited to be part of the ACG family. We share a passion for innovation and the commitment to bring nutraceutical and pharmaceutical customers the best possible solutions and services. By joining forces and leveraging our collective capabilities we’ll no doubt bring Sidebysideä to nutraceutical and pharmaceutical markets the world over.”-Ends-About Vantage NutritionVantage Nutrition, part of the ACG Group, is one of the world’s largest and most respected manufacturer of hard-shell liquid-fill capsule solutions. As a strategic consultancy and manufacturing partner in the nutraceutical space, Vantage Nutrition helps clients bring high-quality and innovative products to market, fast. About ACGIn accordance with its commitment to making the world healthier, ACG has been delivering exceptional solutions to the global pharmaceutical and nutraceutical industry for sixty years, across six continents and in a hundred countries.Collaboration is at the core of ACG’s ethos. ACG is the world’s only integrated pharma manufacturing solutions company, with products ranging from capsules to films & foils, to engineering equipment and inspection systems – all that meet international regulatory requirements. For ACG, it’s always about finding innovative solutions to the world’s greatest health challenges, together.For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more
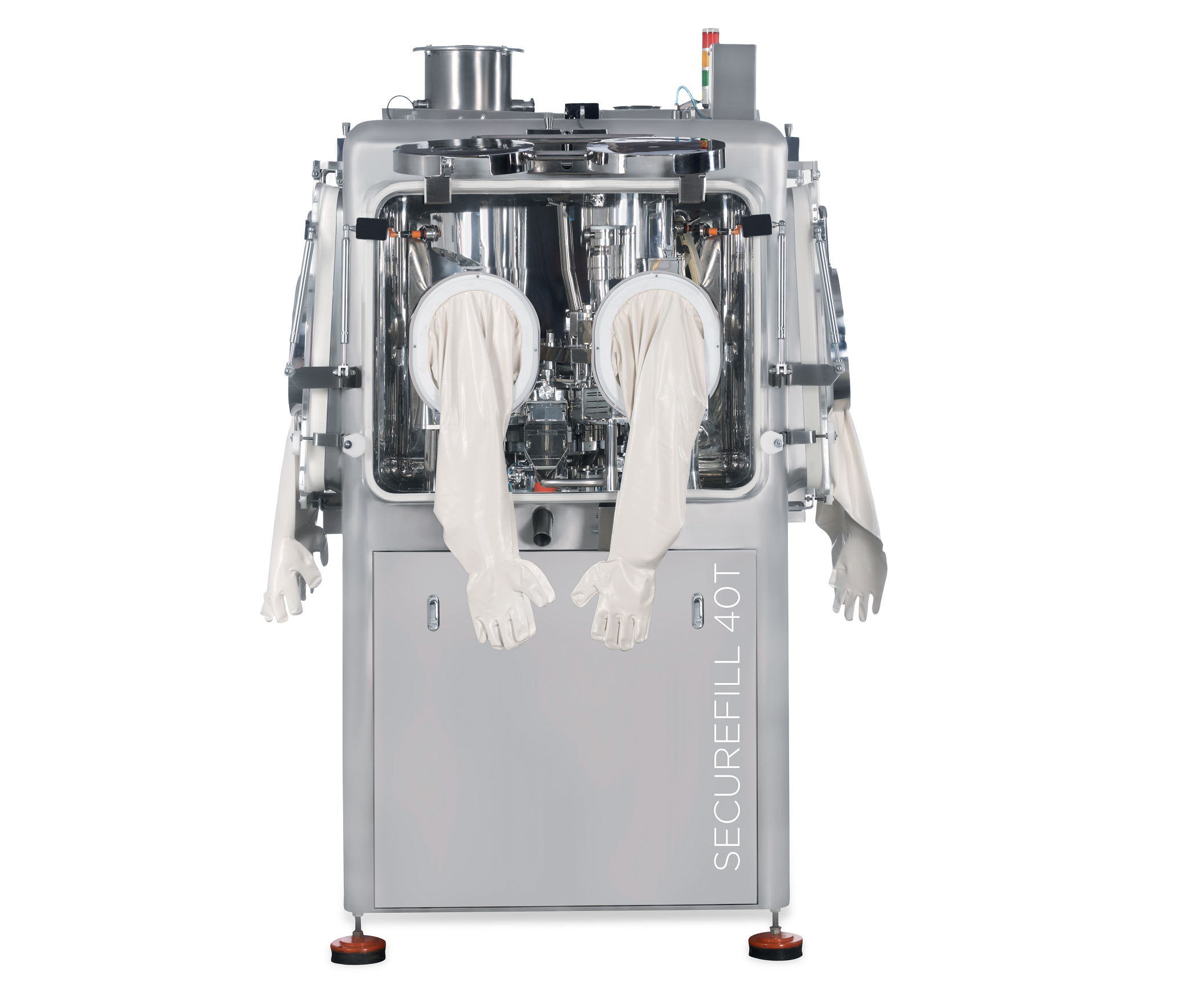
ACG Engineering – SECUREFILL 40T
Tablets & CapsulesCapsule Filling Equipment & Supplies (February 2023)ACG Engineering – SECUREFILL 40TThe Securefill series is ACG’s high-level containment capsule-filling machine range. Designed for filling capsules with highly potent and/or toxic drugs, the machines comply with occupational exposure band (OEB) up to level 4.Built with operator safety in mind, these machines are equipped for filling capsules with oncological, biopharmaceutical, antiviral formulations, and other such highly potent active pharmaceutical ingredients (HPAPIs).Features:• SS316L monoblock structure containing HEPA filters, a rapid transfer port (RTP), glove ports, and accurate dosing mechanisms maintained in a negative pressure environment.• Provision for contained charging and discharging.• Wet-in-place system to ensure wetting of all suspended particles in the pharma zone.• Can be integrated with check-weighers, metal detectors and de-dusters under containment conditions.Benefits:• Ensures complete containment, thereby avoiding operator contact within OEL range 1-10µg/m3.• Integrated containment philosophy for upstream and downstream processes.• Enables easy cleaning.• Supports the production of life-saving drugs that contain HPAPIs.Technical specifications: SECUREFILL 40T Capsule size 00 - 5 Maximum speed 40,000 capsules/hour OEL 1-10µg/m3About ACGIn accordance with its commitment to making the world healthier, ACG has been delivering exceptional solutions to the global pharmaceutical and nutraceutical industry for sixty years, across six continents and in a hundred countries.Collaboration is at the core of ACG’s ethos. ACG is the world’s only integrated pharma manufacturing solutions company, with products ranging from capsules to films & foils, to engineering equipment and inspection systems – all that meet international regulatory requirements. For ACG, it’s always about finding innovative solutions to the world’s greatest health challenges, together.For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

Vantage Nutrition LLC acquires Aquacaps from Nestlé Health Science
Vantage Nutrition’s innovative nutraceutical offering expands into North America.. Vantage Nutrition, an ACG Group company, announced on 1st December 2022 that it has acquired Philadelphia-based ‘Aquacaps’ – an asset of Nestlé Health Science. Aquacaps is a leading contract manufacturer of liquid-filled capsules within the nutritional supplement industry in the United States. Its novel liquid delivery technology allows for the liquid filling of hard gelatin and vegetarian capsules. ACG is the world’s largest integrated supplier of pharmaceutical and nutraceutical products and services. Vantage Nutrition, an ACG group company, already has an excellent reputation as an innovator of two-piece liquid fill capsule solutions. The company’s mission is to be the most efficient partner in delivering innovative and high-quality nutraceutical products to customers globally. Karan Singh, Managing Director of ACG, said: “Although ACG has been established in North America for the last 20 years, this marks our first acquisition in the territory and is a key next step in our global expansion strategy. With this increase in our footprint and manufacturing capabilities, coupled with Vantage’s innovative technologies and 360-degree service offering, we aim to provide the most advanced combination liquid-fill solutions for customers across the region.” About VantageVantage Nutrition, part of the ACG Group, is one of the world’s largest and most respected manufacturer of hard-shell liquid-fill capsule solutions. As a strategic consultancy and manufacturing partner in the nutraceutical space, Vantage helps clients bring high-quality and innovative products to market, fast. About ACGIn accordance with its commitment to making the world healthier, ACG has been delivering exceptional solutions to the global pharmaceutical and nutraceutical industry for sixty years, across six continents and in a hundred countries.Collaboration is at the core of ACG’s ethos. ACG is the world’s only integrated pharma manufacturing solutions company, with products ranging from capsules to films & foils, to engineering equipment and inspection systems – all that meet international regulatory requirements. For ACG, it’s always about finding innovative solutions to the world’s greatest health challenges, together.For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

ACG appoints Shankar Gupta as Chief Sales Officer Industry authority to re-join business in a newly created role
ACG, the world’s largest integrated pharmaceutical and nutraceutical manufacturing solutions provider, is delighted to announce that Shankar Gupta will be re-joining the business in the newly created role of Chief Sales Officer.In his new role, Shankar will be responsible for driving sales across all four ACG divisions (Films and Foils, Capsules, Inspection and Engineering) in order to continue building the company’s ‘One ACG’ agenda. Essentially, this will involve bringing further uniformity to ACG's customer outreach processes, creating more integrated solutions, and ultimately providing a consistent brand experience to ACG’s global customers, regardless of business division or location. He will also focus on scaling new initiatives.Shankar will report directly into the managing director, Karan Singh, who comments:"I am so pleased that Shankar Gupta has decided to rejoin the business during this exciting time. His understanding of ACG and its deliverables, and deep insight into our customers’ businesses will help us to align ourselves better to their changing needs. More importantly, he will champion and support better collaboration and partnership with customers.“Shankar will be pivotal in calibrating and aligning our business units to our goal of ‘Making it Better’, and our belief that everyone deserves access to good medicine."Shankar Gupta adds: “I am excited to use my experience in the pharmaceutical industry to augment ACG's growth by aligning the organisation to the changing dynamics of the global healthcare Industry.“It will certainly call for a huge collaboration of efforts both internally and with our customers, but will ultimately lead to a richer, deeper and more numerous partnerships.”-Ends-About ACGIn accordance with its commitment to making the world healthier, ACG has been delivering exceptional solutions to the global pharmaceutical and nutraceutical industry for sixty years, across six continents and in a hundred countries.Collaboration is at the core of ACG’s ethos. ACG is the world’s only integrated pharma manufacturing solutions company, with products ranging from capsules to films & foils, to engineering equipment and inspection systems – all that meet international regulatory requirements. For ACG, it’s always about finding innovative solutions to the world’s greatest health challenges, together.For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

ACG to Launch German Process Laboratory in 2023
Following the success of its first-to-market laboratory for process development in India, ACG (the world’s biggest integrated pharmaceutical and nutraceutical manufacturing solutions provider) is now also committed to launching a sister process lab in Germany in 2023.This new process development laboratory will be located at ACG’s XERTECS GmbH site in Mulheim in the South West of Germany, with the first phase occupying approximately 250 square metres. ACG’s staffing recruitment plan for the lab will be to attract top academic talent from local universities, as well as trained and experienced process engineers, pharmacists and lab technicians.The aim of ACG’s new laboratory will remain consistent with the first – to provide customers and partners access to the latest knowledge, skills and equipment to collaboratively keep pushing the boundaries of all aspects of process development. So customers can test solid dosage optimisation limits, for example, and co-create alongside ACG’s team of experts to develop new process solutions.The extensive range of available equipment covers areas including feasibility studies, research and development work, pilot-scaling, and tailored in-house technical training courses. Customers also get to experience and test products from ACG’s portfolio and gain operational and maintenance training on premise.Dr Marcus Michel, CEO of ACG Engineering, explains: “ACG’s process labs reinforce our commitment to ‘making it better’. Requested by the Indian market, our first lab has enabled customers to build their competencies and capabilities, as well as to scale up – with many of these customers now operating at high capacity and efficiency. Our plan is simply to replicate this model in the European market, and we are working at full speed to ensure the launch of our new facility in 2023.“The lab in India is home to some of the smartest and finest technologies in powder processing, and is fully equipped for all aspects of granulation, drying, tablet compression and coating. Many of our machines are unique because they have been built or modified in direct response to our customers’ needs – helping them on their manufacturing journey.”About ACGIn accordance with its commitment to making the world healthier, ACG has been delivering exceptional solutions to the global pharmaceutical and nutraceutical industry for over sixty years, across six continents and in a hundred countries.Collaboration is at the core of ACG’s ethos, and ACG is the world’s only fully integrated pharma manufacturing solutions company, with products ranging from capsules to films & foils, to engineering equipment and inspection systems – all that meet international regulatory requirements. For ACG, it’s always about finding innovative solutions to the world’s greatest health challenges, together.For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

ACG appoints Udit K Singh as CEO of ACG Inspection
ACG, a leading supplier of fully integrated manufacturing solutions to the global pharmaceutical and nutraceutical industry, is pleased to announce the appointment of Mr. Udit K Singh as the new chief executive officer (CEO) of its Inspection Division.Mr. Singh will be supporting the company as it continues its growth and global expansion. He will be responsible for ensuring that every dose of medication that ACG Inspection’s pharmaceutical and nutraceutical partners provide is manufactured and delivered exactly as intended. That is down to the fact that ACG’s visual inspection systems track and monitor the manufacturing lines to guarantee flawless, safe products.Karan Singh, managing director, ACG said: “ACG is focused on building a diverse, world-class team by hiring top international talent. We are delighted to welcome Udit Singh to ACG Inspections. He has had an illustrious career and brings diverse and unique capabilities, including a robust understanding of the pharmaceutical sector.“The team looks forward to tapping into his deep experience to enrich our global work towards making the world safer and healthier. With his knowledge and expertise in this field, Udit Singh is sure to take ACG Inspection to newer heights.”Mr. Singh added, “I am pleased to join ACG and will work to ensure that our advanced serialisation, aggregation and anti-counterfeiting solutions track and protect medicines all the way through the supply chain, and into the hands of those who need them.“We are investing substantially to ensure our track and trace platform performs seamlessly and complies with regulatory requirements all over the world. I look forward to playing an integral role, and contributing towards the continued development, working with a world-class team.”About ACGIn accordance with its commitment to making the world healthier, ACG has been delivering exceptional solutions to the global pharmaceutical and nutraceutical industry for almost sixty years, across six continents and in a hundred countries.Collaboration is at the core of ACG’s ethos, and ACG is the world’s only integrated pharma manufacturing solutions company, with products ranging from capsules to films & foils, to engineering equipment and inspection systems – all that meet international regulatory requirements. For ACG, it’s always about finding innovative solutions to the world’s greatest health challenges, together.For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

ACG Capsules launches campaign to promote problem-free pharmaceutical production
-One of the world’s largest producers of empty hard-shell capsules is on a mission to‘Love NOTHING’ when it comes to zero-problem productionACG, one of the world’s largest producers of empty hard-shell capsules, has launched its global pharmaceutical campaign: ‘ACG loves NOTHING’. The campaign is aimed at significantly improving pharmaceutical production through collaboration and industry-leading innovation. With a host of value-added services on offer, ACG has taken a pledge to strive for zero-problem pharmaceutical and nutraceutical manufacturing.Alex Robertson, CMO at ACG, said: “The messaging at the centre of this campaign is: ‘ACG loves NOTHING’ – we are putting every effort towards zero-problem production. That’s because when ‘nothing’ happens during pharmaceutical and nutraceutical production, everything runs reliably, and our customers can get the products they make to the people who need them. We will achieve this by providing the highest quality empty capsules and all the holistic advice and expertise required for seamless operations.”Founded 60 years ago, ACG was created on the simple principle of ‘Make it Better’. As the world’s largest integrated supplier of solid dosage pharmaceutical and nutraceutical products, and with the most comprehensive ranges of vegetarian and gelatin capsules globally, ACG has the scale and range to have a far-reaching impact on pharmaceutical production and human health.Selwyn Noronha, CEO ACG Capsules, added: “The pharmaceutical sector is regulated and highly competitive. Companies operating within it need a diverse range of quality capsules that help them stand out from the crowd, while also meeting demands for clean and natural products.“Beyond exceptional products, these companies need expertise and a lifetime of targeted support to ensure efficient manufacturing and guarantee regulatory compliance. With proficiency in all aspects of manufacturing from capsules to machines and protective barriers, for the last 60 years ACG has been the only company to offer this level of support, and it continues with this focus at the heart of operations.”So, at ACG, there really is something about NOTHING. The company is on a mission to drive towards problem free production and collaborating with its customers to collectively deliver on its mission of creating a healthier world.About ACGIn accordance with its commitment to making the world healthier, ACG has now been delivering exceptional solutions to the global pharmaceutical and nutraceutical industry for 60 years, across six continents and in 138 countries.‘Collaboration’ is at the core of ACG’s ethos, and ACG is the world’s only integrated pharma manufacturing solutions company, with products ranging from capsules to films & foils, to engineering equipment, and inspection systems – all that meet international regulatory requirements.For ACG, it’s always about finding innovative solutions to the world’s greatest health challenges, together.For more information, please contact the ACG media relations team:madhurima.chakraborty@acg-world.com
Read more

What is pelletisation, and how is it advancing nutraceuticals and dietary supplements?
Pelletization has attracted much attention across the nutraceutical sector. Experts from ACG explain why.Nutraceuticals are gaining popularity as an effective tool for maintaining health as public awareness of the advantages of health supplements grows. According to market analysis, the global nutraceutical segment is anticipated to increase in value from $450 billion in 2021 to $746 billion by 2028, with a projected compound annual growth rate (CAGR) of around 8.8%.1A variety of products are now included within the nutraceutical category. Companies are implementing new delivery systems to further expand their nutraceutical ranges. Many are taking inspiration from pharmaceutical manufacturing and adapting these innovative techniques for the delivery of nutraceutical products.Tablets and capsules are the most common formats for pharmaceutical and nutraceutical oral delivery systems. Currently, these formats are undergoing different modulations. In particular, many are adapting one of the most appealing technologies used in controlled drug delivery: pelletization.Pelletization and NutraceuticalsPelletization has attracted a great deal of attention across the sector. It is now being applied to nutraceuticals in the same way as pharmaceuticals, to alter the release pattern of formulations.The pelletization process formulates active ingredients in free-flowing spherical beads, granules, or pellets, which can be coated to provide the required modified-release properties. Additionally, this process offers numerous other benefits to manufacturers, including the ability to combine incompatible components in a single dosage form.There are different methods for pelletizing active ingredients, including: extrusion spheronization, fluidized bed coating, dry powder layering, and spray congealing.The pellets prepared in the process are either made directly from the active nutraceutical ingredients (ANI), or they can be base pellets that act as a vehicle for later stacking of active material. A product may comprise 90% of the ANI along with the additives, or base pellets.Two types of base pellets are used for stacking. The first are neutral starter pellets, which are inert and usually used to avoid interaction with the ANI. Examples include sugar spheres, MCC (microcrystalline cellulose) spheres, wax-based spheres, silicon dioxide spheres, lactose-starch/lactose-cellulose spheres, and rice-based spheres. The other type of base pellets are functional starter pellets that actively promote formulations by enhancing the ANI’s solubility, dissolution, and stability.Pellet preparation enables the formulator to alter the product’s properties and release pattern. It is possible to incorporate immediate- and controlled-release delivery mechanisms in pellets by using fast-disintegrating pellets and pellets coated with different polymers, respectively. For ingredients with poor solubility, pellet techniques help to deliver the desired effect by combining excipients that enhance solubility—for example, curcumin pellets. Additionally, it is possible to achieve sustained release by coating the pellets, as in the example of caffeine pellets.Encapsulation of Nutraceutical PelletsThe most effective way to deliver pellets in the smallest container is in capsules. Many nutraceutical manufacturers adapt the capsule dosage form to deliver their product, as it provides better tolerance over other dosage forms. Capsules also serve as site-specific and prolonged-delivery systems. For example, sustained-release caffeine pellets in a hard capsule can slowly release caffeine into the body over a prolonged period, offering a sustained release of the caffeine’s effect.There are further examples of active nutraceutical ingredients formulated in pellets and encapsulated in hard capsules which are currently on the market. These include:Soy protein pellets in capsulesEmblica officinalis (amla) pellets in capsulesAsparagus racemosus (shatavari) pellets in capsulesExamples of HPMC (vegetarian) capsules:Zinc pellets in capsulesCrocus sativus (saffron) pellets in capsulesVitamin B6 pellets in capsulesPellets generally offer an advantage over other oral dosage forms, in the effective administration of multiple incompatible materials at the same time. It is possible to combine two or more release patterns in one capsule as pelletization is adopted. Additionally, it is feasible to incorporate two types of pellets with the different ANIs—in their active form alongside liquid in a single capsule.The demand for manufacturing technologies that overcome formulation issues such as stability, absorption, and permeability has increased because the market requires advanced formulations. For nutraceutical products, it is difficult to add two incompatible active ingredients in a dosage form such as a tablet; however, two different pellets in a hard capsule can resolve this challenge. By doing this, a synergistic therapeutic effect can be achieved using two different types of pellets. Nutraceutical formulations with combination fills may come in the form of liquid + pallets or liquid + capsules, allowing two incompatible active ingredients to be combined and have a synergistic effect.In particular, the liquid + pellet combination dosage form can produce various profiles, such as immediate release dissolved in liquid and delayed release in pellet form. For example, take a complex pellet containing vitamins A, D, E, and B. It is also possible to add two different nutraceutical pellets combined with liquid nutraceuticals for combination therapy.Another combination-fill technique involving liquid + capsule helps to minimize stability issues. It is the most common method for combination fill and consists of one active ingredient dissolved in the carrier solvent and the other, in pellet form, enclosed in a smaller capsule that is dispersed in the liquid. For example, with vitamin A, E, and C capsules, the capsule can contain the nutraceutical active ingredient in pellet form to further aid the sustained release of the ANI.Advantages of PelletizationThere are definite advantages to pelletization techniques and formulation. Pelletizing active nutraceutical ingredients can help improve the efficacy of a formulation by modulating the solubility and bioavailability of lipophilic and moderately-water-soluble active ingredients. It is also possible to achieve sustained release for the active ingredient by formulating it in pellets. The therapeutic effects of the ANI can be enhanced by the pellet formulation as the pellet disintegrates in the GI system.There are additional advantages. Nutraceuticals with an unpleasant taste can be masked in pellets to make a product more palatable and increase consumer compliance. And using colored coating material to manufacture the pellets will not only improve the appearance of the formulation but will also provide the product with uniqueness to appeal to consumers and provide brand recognition. This color mix and the aesthetic appeal of the pellet-embedded capsules can attract consumers and, in turn, support product marketing.HPMC Capsules: A Vegetarian AlternativeIt is feasible to encapsulate nutraceutical pellets in gelatin (HPMC, hydroxypropyl methylcellulose) or hypromellose capsules; however, HPMC is considered the better option as many of the challenges faced by nutraceutical manufacturers, mentioned below, can be overcome with these capsules.The major challenge for nutraceutical providers is maintaining the stability of the compound and the overall aesthetic of the product. Many of the vitamins (vitamin C, thiamine, vitamin B12, pantothenic acid), amino acids (acetyl-L-carnitine HCl, L-arginine base), enzymes and co-enzymes, and certain minerals and their active salt forms are moisture sensitive, and HPMC capsules offer the better final product owing to its low inherent moisture content. The growing trend in vegetarian and clean-label capsules makes the HPMC capsule a safe choice.SummaryPelletization is a game-changing technology for the nutraceuticals market. Enabling a wide range of delivery formats, along with customer compliance, are inherent benefits making it the delivery system of choice for many nutraceutical manufacturers. This technique is leading the way to the development of novel delivery systems, which create new and emerging opportunities for the sector.About the authorsJnanadeva Bhat, PhD, is head of formulation R&D (Pharma & Nutra) at ACG Group. Bhat has been associated with the pharmaceutical industry for more than two-and-a-half decades. As a product formulator, he has worked on various dosage forms that include tablets, soft gelatin and hard capsules, injectables, and lyophilized formulations. At ACG, he heads the formulation R&D lab where he primarily leads new product development projects and customer interface.Manali Dalvi, lead, white papers, formulation R&D (Pharma and Nutra), is part of the Capsules R&D team at ACG. Her primary responsibilities include writing and publishing scientific research articles and developing segmented solutions and technical content as part of thought-leadership programs. She is also involved in all of the company’s industry- and institute-related collaborations and research activities.ReferenceGrowth Scope of Nutraceuticals Market Size Worth USD 745.5 Billion by 2028 at 8.8% CAGR- Industry Trends and Forecast Report by Zion Market Research. News release. PR Newswire; April 20, 2022. https://www.prnewswire.com/news-releases/growth-scope-of-nutraceuticals-market-size-worth-usd-745-5-billion-by-2028-at-8-8-cagr---industry-trends--forecast-report-by-zion-market-research-301529003.html Source: https://www.nutritionaloutlook.com/
Read more

Advanced manufacturing will pave the way for the pharmaceutical industry
The time has come to transition to sustainable strategies centred around inclusion, innovation and integrationAdvanced manufacturing will pave the way for the pharmaceutical industryKaran Singh (pictured), Managing Director at ACG, says: 2023 will continue to pave the way towards sustainable manufacturing, with a growing number of organisations placing focus on creating long-term solutions.Now is the time for new manufacturing sites to adopt and implement new technologies and to transform pharma manufacturing from being reliant on costly legacy processes.Digital technology will speed up processes by enabling early detection of wear and tear on machinery lines. It will also help to reduce wastage and recalls by effectively tracking defective batches, allowing for timely interventions.Placing digital "Industry 4.0" technologies – such as AI, machine learning, IoT, Augmented and Virtual Reality – at the heart of manufacturing can further improve R&D and speed up testing, compliance and efficiency.Adopting Industry 4.0 technology and new delivery models can help to build a more self-reliant and robust pharmaceutical supply chain to keep up with changing and increasingly complex healthcare needs.Our focus is on creating high-performing and quality driven manufacturing units that can support smart, connected, and intelligent systems. Reams of data converts into actionable intelligence, improving several supply chain aspects such as inventory management, trend analysis, forecasting and predictive equipment maintenance.The growth of digital health technology and telemedicine will continue to drive healthcare innovations in 2023.Developments such as medical device integrations and software monitoring will be centred around at-home and remote diagnosis, ultimately aimed at improving clinical outcomes and pre- and post-consultation care for more ‘informed’ patients. Inclusion, innovation and integration will be keywords for the pharmaceutical industry in 2023Source: https://www.manufacturingchemist.com/news/article_page/Advanced_manufacturing_will_pave_the_way_for_the_pharmaceutical_industry/206066
Read more

European Pharmaceutical manufacturer
Top pharma industry trends to watch for in 2023
EPM conducted a roundtable with several pharma experts to learn what industry trends to watch out for in 2023, from AI drug discovery through to the rise of decentralised trials.Tim Guilliams, CEO and co-founder, Healx:"Thanks to improvements in software and computing, the field of drug discovery is being completely reimagined with life changing effects for the hundreds of millions of people living with rare diseases worldwide. In 2023 we will increasingly see AI used across the entire drug discovery and development pipeline. From de novo design of molecules and treatment combination predictions, to preclinical validation, right up to the running of clinical trials for precision medicine. We will also see greater collaboration between industry, technologists, academics and patient groups to progress more novel rare disease treatments towards clinical trials."Christian Dowdeswell, vice president, head of Commercial Development, Small Molecules, Lonza:"The ongoing trend of new drugs becoming ever more complex is sure to continue. The number of synthetic steps for making small molecule APIs has grown by two-thirds in the past 20 years, as has the number of chiral centres. Average molecular weights continue to rise, and maybe three-quarters have poor solubility. With so many NMEs being approved via some form of expedited approval pathway, there is bound to be continued high demand for CDMO services, and a demand to meet ever-shorter timelines. Innovation will be vital, and digital tools that support the development of robust processes will be increasingly important.Ambitious targets have already driven significant sustainability advances, many of them remediating older processes developed when it was less of a concern. We now embed sustainability into our process design for chemical synthesis, and specific projects look to eliminate toxic materials from a process, or replace environmentally damaging chlorinated solvents in spray drying. I fully expect this will become a key area of differentiation in the CDMO industry."Kyle Cunningham, chief product officer, Greenphire:“Clinical trial sites face a host of challenges when it comes to keeping trials moving along. While technology is designed to benefit sites, they often feel overwhelmed by the sheer number of technologies that are required for a given trial. In 2023, I believe we will see a concerted effort from sponsors, CROs and solution providers to integrate technology platforms and workflows. For example, sites will benefit from data-driven processes, such as participant payments being automatically triggered via EDC data or completion of participant activity within their ePRO. Through the integration of site platforms, we can eliminate administrative burdens, increase efficiency and empower sites to provide better care and support for their participants.” Jim Murphy, CEO, Greenphire:“Over the past few years, we’ve heard a great deal about the rise of decentralised trials (DCT). In 2023, I predict that the industry will focus less on DCTs and place a heavier focus on strengthening the site-patient relationship. The site will remain the central stakeholder of a clinical trial but with a greater emphasis placed on the notion of heightened flexibility for the participant. We will see further adoption of innovative tools that offer options related to receiving care and completing trial activity while keeping the site in control and enabling them to maintain and even increase efficiency. By empowering sites to better support their participants through versatility of care, we as an industry can drive towards stronger recruitment efforts, greater engagement and improved retention, resulting in overall trial optimisation.”Jim Lehane, global leader of Life Sciences Manufacturing, Cognizant:"Biopharma manufacturers are constantly creating an abundance of data. The sheer volume of data can be a blessing and also a curse. In order to create actionable insights and proactive decision making, data strategies need to be in place to capture, analyse, utilise and protect it throughout the integration of technology. When handled correctly, insights can become extremely valuable intellectual property (IP) that can drive significant business innovations and give a competitive advantage. PHILLP CULLINANE The adoption of new technologies into manufacturing processes is the crux of major innovation across the sector. Integration of digital technologies and shared interoperable platforms are helping the biopharma industry improve operational efficiency in supply chain management post-pandemic. A big part of this trend is the engagement of automation across the enterprise, merging data from information technologies and operational technologies (OTs), connecting systems and processes to create a seamless transition from drug discovery to commercial batch release.The life science industry of yesterday kept data siloed within different parts of the business, effectively keeping it “locked up” and limiting its value. Now, the industry is trying to release the intrinsic power of data from the IT and OT layer utilising cloud technologies – with data strategies built in at design, creating a single source of truth. This approach means data can be leveraged to deliver real-time insights and improved business outcomes through efficiency, reduced supply costs, and increased quality.But this desire for digital transformation has raised a prominent challenge: technology is becoming increasingly complex and many companies lack the experience and expertise needed to ensure the security, efficiency, and success of any digitalisation or automation initiative.Relevant experience within the industry and the technology-based evolution of manufacturing will therefore be increasingly necessary to ease biopharma’s transition into the digital age and provide flexibility as industry requirements change. Manufacturers throughout the life science industry that successfully integrate advanced digital and automation technologies, with a clearly defined data strategy will be well-positioned to ensure patients continue to have access to critical medicines."Sara Lesina, MBA, general manager Business Unit Europe, Sirio Europe:"Nutritional science is advancing its understanding of the chemistries and therapeutic potential of a myriad of different nutritional compounds and extracts. But it is important to remember the entire category is driven primarily by consumers, who are increasingly selective about the supplements they buy. Consumers want their nutraceuticals to possess five fundamental things: taste and texture, health benefits, be free from additives, environmentally friendly and non—GMO. They also want them to be easy to chew and swallow and affordable. Erik van der Burgt / VRBLD photofilmStudent van Vlerick Business School.There was a time when tablets and capsules were perfectly suited to the vast majority of products on the market, but consumer preferences and science continue to move the industry away from an overreliance on traditional oral solid dose forms. Increasingly that means delivering products in ways that people prefer. Softgels and the rising champion, the gummy, are two forms gaining significant ground with product designers and consumers. One look down any nutritional supplement aisle in any grocery store or pharmacy in the world will support that assertion.For consumers, dose form really matters. Pill fatigue is a real phenomenon especially among older patients. No matter how effective the active ingredient may be, it will never deliver its therapeutic effect if it is not taken as prescribed or as recommended. To attain those mass-market product goals and meet consumer demands, the industry will be increasingly prompted to access more application science from its external pharmaceutical-grade manufacturing partners."Tony Page, SVP Insight Analytics, Within3:“As we look to the year ahead, companies must lean further into what worked. Be wary of complacency – organisations that are content with the status quo will be outperformed by those who prioritise areas where they can see the most acceleration. This means elevating insights management to a main strategic pillar. Whether it’s cutting through masses of data or reducing the time it takes to analyse data sets, creating efficient workflows and solving the life science insights gap is critical. In the year ahead, we’ll also see life science leverage artificial intelligence even more strongly, with AI supporting tech-enabled team members rather than replacing them.”Chuck Miller, director of Solution Consulting, AspenTech:"What good is taking operations digital if the existing data streams offer limited value? Today’s operating units generate a richness of process data, but this data is not always deemed sufficient for critical monitoring, control and release workflows, especially in the case of legacy operating lines. The effectiveness and reliability of Process Analytical Technology (PAT) has improved greatly in the past several years. Taking advantage of this to enrich operations data streams enables faster commercialisation, more cost-efficient production and reduced supply lead time."Cathy O’Brien, vice president for International Sales, UPS Healthcare:“Sustainability is, and will continue to be, a critical issue for the industry. Nearly three quarters of the industry’s emissions come from Scope 3 – activities like transport and packaging – which sit outside of the pharma company’s direct control.A further challenge is the need to balance the availability of energy and the ongoing inflationary costs with quality of supply, in what will be a very dynamic macroeconomic and geopolitical environment.Public-private partnerships will continue to be crucial, much like we saw for Covid vaccine development and distribution, to create the scalable, sustainable and cost friendly solutions that can work across the industry.80% of pharma products in Europe already require temperature-controlled transportation, and that number is only going to grow. With that growth comes the need for next-generation products and services to suit the transport and storage of next-generation healthcare treatments. This means innovation throughout the supply chain to ensure every pharma company has an end-to-end solution that offers unprecedented control, visibility, and reliability. The fragility and high value of these new drugs, like biologics, means that it’s now more important than ever that the industry knows where a shipment is at all times and that it will arrive on time and at the right temperature."Terry Lo, president and CEO, Vizgen:"We’ve already seen an explosion of AI approaches being utilised for drug and biomarker discovery. I anticipate this trend will only continue in 2023. With new platform technologies emerging, there is an increasing density of the data generated from a single sample. The payoff in using AI and more efficient analysis tools before entering clinical trials will become even more dramatic because of the richness of the starting data.Also, massive amounts of data being a challenge in 2023 is a pretty safe prediction. The increasing amount of data is not just the addition of multi-omics, but the combination of high parameter content alongside high resolution imaging. Spatial genomics is a great example, where you can detect the precise location of over a billion transcripts in a single square centimeter of tissue sample. The challenge here is terabytes of data are generated from that single sample."Karan Singh, managing director, ACG:“2023 will continue to pave the way towards sustainable manufacturing, with a growing number of organisations placing focus on creating long-term solutions.Now is the time for new manufacturing sites to adopt and implement new technologies; to transform pharma manufacturing from being reliant on costly legacy processes. Digital technology will speed up processes by enabling early detection of wear and tear on machinery lines. It will also help to reduce wastage and recalls by effectively tracking defective batches, allowing for timely interventions.COLSTON JULIAN ColstonJulian Placing digital ‘Industry 4.0’ technologies – such as AI, machine learning, IoT, Augmented and Virtual Reality – at the heart of manufacturing can further improve R&D and speed up testing, compliance and efficiency. Adopting Industry 4.0 technology and new delivery models can help to build a more self-reliant and robust pharmaceutical supply chain to keep up with changing and increasingly complex healthcare needs. Our focus is on creating high-performing and quality driven manufacturing units that can support smart, connected, and intelligent systems. Reams of data converts into actionable intelligence, improving several supply chain aspects such as inventory management, trend analysis, forecasting and predictive equipment maintenance.The growth of digital health technology and telemedicine will continue to drive healthcare innovations in 2023. Developments such as medical device integrations and software monitoring will be centred around at-home and remote diagnosis, ultimately aimed at improving clinical outcomes and pre- and post-consultation care for more ‘informed’ patients.Inclusion, innovation, and integration will be keywords for the pharmaceutical industry in 2023.”Source: https://pharmaceuticalmanufacturer.media/
Read more
Considerations for Manufacturing Solid Versus Semi-solid Drugs
Manufacturers must consider key components of manufacturing solid versus semi-solid drugs to create a successful end product.When it comes to solid vs. semi-solid drugs, there are manyconsiderations for manufacturers to take in regards to the process in which the end product is made. While solid dosage drugs may be easier to manufacture, they present more challenges regarding regulatory requirements. While semi-solid dosage drugs are more challenging to manufacture, they are subject to fewer regulatory obstacles.To learn more about the manufacturing processes for solid and semi-solid dosage drugs, Pharmaceutical Technology spoke with Shankar Gupta, chief sales officer at ACG. Solid versus semi-solid drugsPharmTech: What are the pros and cons of solid dosage versus semi solid dosage, or capsules vs. soft gels?Gupta: Each of them have their own pros and cons. On the one hand, from a manufacturing standpoint, capsules are fairly straightforward. The capsules are filled using a filling machine,and the product is ready. However, there are a number of standard and regulatory requirements that need to be met before they are approved for consumption and marketing.Although soft-gel capsules are subject to fewer regulations, the manufacturing process is both longer and more complex, requiring a larger manufacturing space. Additionally, the processes require greater numbers of staff with a wide range of skills. Also, processes result in more wastage. Before they can be packed, they [also] need to be dried, which adds to the time, complexity, and cost.Key considerationsPharmTech: How can the formulation of a drug impact the quality of the resultant oral solid dosage form and the tableting process altogether?Gupta:The formulation of a drug is governed by a number of key factors. For a contract manufacturer, this would be determined by the customer’s preference or need. Otherwise, the formulation of a drug is generally dictated by the patient’s experience, compliance, and the appropriate packaging for the product.In the case of needing to taste mask to achieve a neutral product or to add color, capsules would be the preferred choice.Although capsules have a good shelf life, when exposed to humidity, temperature fluctuations, or if they are subjected to prolonged light exposure, they can become brittle. Despite these variances, the packaging for capsules is quite simple.[On the other hand,] tablet packaging has a higher degree of complexity due to varied shapes, sizes, and consistency of the end product.PharmTech: What processes can be used to ensure uniformity/equal distribution of ingredients in capsules/tablets during manufacturing?Gupta: From a mechanical perspective, the machinery utilized to ensure the uniformity or equal distribution of ingredients depends on the requirement of the formulation, and also determines the process. For example, in cases of a high degree of content uniformity, the processes may rely on the utilization of a fluid bed drier, processor, or a high-shear mixer.Other dependencies include the dissolution time, [need] for fast delivery, or the disintegration requirement.PharmTech: Can you explain tablet tooling and provide some considerations to make when deciding what methods to use?Gupta: Tablet tooling refers to a tooling method that uses upper and lower punches, and compression force with a die and cup to shape granules or powder into a tablet. The die is used in conjunction with the punches to accept the granules or powder for compression and creates the tablet’s perimeter size. The cup depth determines the tablet’s depth.The end product will influence the tooling parameters including the tablet size, based on economies and end-user price point. This goes from low-cost packaging through to the creation or innovative shapes to drive product differentiation and competition in a particular category and market.Challenges and trendsPharmTech: What are some of the main challenges drug manufacturers face when trying to process oral solid dosage forms/tablets quickly and cost-effectively while also maintaining high quality?Gupta: It is widely known that efficiencies in pharmaceutical productivity are not comparable to productivity gains in other manufacturing sectors. Running at an efficiency of 60–65%, the biggest challenge across the sector is by far productivity.Also, as accuracy and quality are vital in cases where products are exported to regulatory markets, the levels of inspection are hugely increased. This, in turn, can hamper the ability to streamline and drive efficiencies.The other challenges facing pharmaceutical manufacturing are varied, from API variations to labor shortages, speed of product inspection, production timelines, and speed of adopting new technology. However, the industry is accelerating its digital Industry 4.0 transformation in pursuit of data-controlled processes, which should help the sector to increase efficiencies, as well as speed to market.PharmTech: Have you witnessed any significant trends in tableting over the past decade that have had a positive/negative impact on the industry?Gupta: We are seeing a rise in multi-unit pellet system tablets. This is a solid dosage form that is the outcome of compressing a mixture of pellets that contain drugs and powder excipients [in order] to control drug release.We have also seen a significant change in the adoption of inspection technology. The COVID-19 pandemic made it more evident that human inspection regarding the quality of products must be reduced, for example, by removing processes such as hand-picking products to test. Companies that focus on cost control, through the use of more efficient equipment and less onerous inspection processes, will not only reduce waste and the need for costly labor, but will also benefit from a reduction in costly product recall.Manufacturers who adopt inspection technology benefit from microlevel distinction differentiation of the product; inside and outside. These inspections are efficient performing at high speed and in real-time, meaning samples do not have to be taken off the line to be tested. Inspection technology assesses every single product and provides real-time intelligence regarding rejections so the quality of the products in the batch that haven’t been rejected can be guaranteed.Where as the sample method of testing means the entire batch quality is based on the test results of a subset of the batch and the remaining products in the batch are assumed to be of the same quality but can’t be guaranteed.PharmTech: Could you give us your predictions on the trends within tableting for the coming decade?Gupta: A growing and ageing global population, along with new emerging markets, are creating high demand in the pharmaceutical sector globally. With an increase in competition, speed to market is vital. Those who are customer-centric, provide safety assurance, and deliver faster will ultimately win.We are seeing the nascent signs of demand for high-output machines. This is coupled with the need to drive efficiencies in the sector. The combination of advancements in Industry 4.0 and the need for leaner workforces will drive the analysis of multiple sources of data to provide information such as predictive maintenance and even remote maintenance. Where machine uptime and efficient running costs are improved, cost savings will be achieved without compromising the quality of the product.Additionally, we are starting to see a greater uptake in 3D printing in the sector. Being able to print in small batches results in waste reduction, coupled with reliable outputs and efficiencies. It will have a useful place in the set-up of new product development and has the potential to revolutionize the delivery of personalized medicine.About the authorAlivia Leon is assistant editor at Pharmaceutical Technology.Article detailsPharmaceutical TechnologyVol. 47, No. 1January 2023Page: 30-32CitationWhen referring to this article, please cite it as A. Leon, "Considerations for Manufacturing Solid Versus Semi-solid Drugs," Pharmaceutical Technology 47 (1) (2023).Source: https://www.pharmtech.com/
Read more

2023 Pharma Trend Outlook: Innovation, Resilience, and Pharma 4.0
Download our 2023 Pharma Trends Outlook report to discover the trends set to shape the pharmaceutical landscape in the new year, with expert opinions and insight from across the pharmaceutical value chain.As we welcome in a new year with new possibilities, CPHI Online is proud to bring you the 2023 Pharma Trends Outlook report in partnership with ACG World.This Trend Report explores 12 trends predicted to shape the pharmaceutical landscape in 2023 and the implications they will have on all pharmaceutical players, including suppliers, research and development, manufacturing, and distribution.The pharmaceutical industry has witnessed a great many innovations and advancements in the last year. With 32 novel drug approvals to date, a bivalent COVID-19 vaccine, and the return of face-to-face events, the industry is primed to see the continuation of these positive developments. However, issues including supply chain disruptions, rising inflation, and global events such as the War in Ukraine have presented unprecedented challenges for the pharmaceutical industry, leaving all players feeling the pressures of these events.In this Trend Report, we cover trends such as Pharma 4.0 and the Data Revolution, sustainability trends, pharmaceutical start-up initiatives, cell and gene therapies, and more with expert insight from across the pharmaceutical value chain. As we enter a post-pandemic world and face new global challenges, the pharma industry must be applauded for its pursuit of new products, technologies and solutions which ultimately improve the lives of people worldwide.Register and download our full Trend Report to see what is in store for the pharmaceutical industry in 2023. Source: https://www.cphi-online.com
Read more

Ajit Singh
Ajit Singh earned his graduation degree from Cambridge University, UK, and post-graduation degree from Harvard Business School, USA. He currently resides in Mumbai, India, and travels widely to study pharmaceutical and allied industries.
Ajit is the Chairman of the ACG Group (formerly known as the Associated Capsules Group of Companies), which is headquartered in Mumbai. He has played a prominent role in the development of the pharmaceutical and allied industries in India and has served as an executive committee member of the Federation of Indian Chambers of Commerce & Industry (FICCI), a regional council member of the Confederation of Indian Industry (CII), Western Region, and other prominent pharmaceutical associations in India. He has also been conferred with prestigious awards for his contributions and thoughtful leadership to the pharmaceutical industry.
Ajit was a founder-member of the Young Presidents’ Organization – India Chapter and the first Indian to be invited to serve on its international board in the USA.

Jasjit Singh
Along with his brother, Ajit, Jasjit started the Associated Capsules Group in Mumbai in 1962.
Jasjit has always been interested in technology – particularly that relating to machine design – and earned a degree in Mechanical Engineering from London University. He has dozens of patents to his credit, and under his leadership the ACG Group has received numerous global awards for technology, excellence and social welfare.
Jasjit has been actively involved with starting new Forums of the Young Presidents’ Organisation. He served as the Chairman of the Mumbai Chapter, and helped found the Kolkata Chapter before becoming a founding President of the World Presidents’ Organisation. Jasjit has also received the Vijay Ratna Award for leadership in Industry, and recognition from UNIDO for his contribution to the fields of pharmaceutical technology and environmental science.
Jasjit’s other interests include self-education, reading, music, collecting rare works of arts and helping young contemporary artists, cooking, and travelling to different parts of the world – especially with his family.

Karan Singh
An organisational psychologist by education, Karan has transformed ACG from a modest Indian business into a world-renowned institution, revolutionising its vision and long-term strategy to ensure global growth. Karan has overseen ACG’s remarkable digital transformation, adopting breakthrough technologies that not only keep medicines safe, affordable, and accessible – but also vastly improve manufacturing and supply chains. Meanwhile, he has led a series of tuck-in acquisitions that ensure ACG pioneers continuously in terms of innovation and scale.
Passionate about converting ideas into growth opportunities, he supports entrepreneurial visions that solve real-world challenges. As an investor and mentor to more than 35 health-tech start-ups, guiding strategy, technology, expertise and networks, Karan seeks to consolidate the next generation of leadership – one that will secure better access and bridge availability across the global health industry.
Karan serves on the India Advisory Board for the India-Brazil Chamber of Commerce, is Co-Chair for FICCI LAC Regional Council, a key advisor to Nasscom, and an active contributor to the Global Lighthouse Network community of the World Economic Forum. Years of keen basketball-playing underpin his leadership, communication, and competitive spirit, and have brought about ACG-NBA Jump, a grassroots programme to give Indian basketballers a global platform.

Selwyn Noronha
CEO ACG CAPSULES

Selwyn Noronha

Richard Stedman
CEO ACG ENGINEERING

Richard Stedman

SR Shivshankar
CEO ACG PACKAGING MATERIALS

SR Shivshankar

Udit Singh
CEO ACG INSPECTION

Udit Singh

Sunil Jha
Chief Human Resource Officer

Sunil Jha

Werner Bongers
CEO SCITECH

Werner Bongers

Nitin Desai
Chief Commercial Officer

Nitin Desai

Parag Shah
Chief Financial Officer

Parag Shah

Shankar Gupta
Chief Sales Officer

Shankar Gupta

Balajikasiram Sundararajan
Chief Digital Officer

Balajikasiram Sundararajan

Alexander Robertson
Chief Marketing Officer

Alexander Robertson

Boilerplate
For over 60 plus years, ACG has been innovating the production solutions for pharmaceutical and nutraceutical companies, that help make people better.
As the world’s most integrated provider of oral dosage products and services, we produce capsules, barrier packaging materials, manufacturing machinery, and visual inspection and traceability solutions. All fully compliant with international standards.
Today, ACG fosters long-term collaborative partnerships with customers in 138 countries across six continents.
Together, we share a common purpose: to solve the world’s greatest health challenges and make it better for everybody we serve.