The evolution and future of inhalation drug delivery systems
The respiratory system's unique anatomical and physiological characteristics make it an ideal target for both local and systemic drug delivery. The lungs present an expansive surface area coupled with high vascularity, enabling efficient drug absorption without the complications of first-pass metabolism. This allows for significantly lower dosages compared to oral administration while maintaining therapeutic efficacy.
Consider the case of salbutamol (albuterol), where inhalation delivery requires merely one-tenth of the oral dose – 0.2-0.4 mg compared to 2-4 mg orally. Moreover, the onset of action through inhalation is remarkably faster, typically around five minutes, versus thirty minutes for oral administration. This rapid response time can be crucial in managing acute respiratory conditions.
The efficiency of drug delivery through inhalation typically ranges between 12% and 40% of the inhaled dose, with the remainder being expelled or deposited in the upper respiratory tract. However, the targeted delivery of the drug at the site of action allows for lower doses compared to oral administration, resulting in reduced side effects and improved patient outcomes.
Current technology landscape
The inhalation drug delivery landscape encompasses three primary technologies, each with distinct characteristics and applications. Traditional nebulisers, while effective, are primarily confined to hospital settings due to their size and complexity. Their requirement for professional supervision limits their application in routine chronic disease management. However, they remain valuable in specific clinical scenarios where precise dose control is crucial.
Pressurised metered dose inhalers (pMDIs) contain drugs dissolved or suspended in a propellant. When the device is activated, the aerosolised drug is delivered to the lungs. However, the environmental impact of the propellants used in pMDIs has raised significant concerns. Although these devices have evolved, transitioning from the use of chlorofluorocarbons (CFCs) to hydrofluorocarbons (HFCs), the HFCs currently in use have been identified as greenhouse gases. Efforts are underway to develop and introduce less harmful HFC propellants to the market. Besides these, other challenges persist, particularly dose uniformity issues and coordinating the actuation of the device with inhaling the medication.
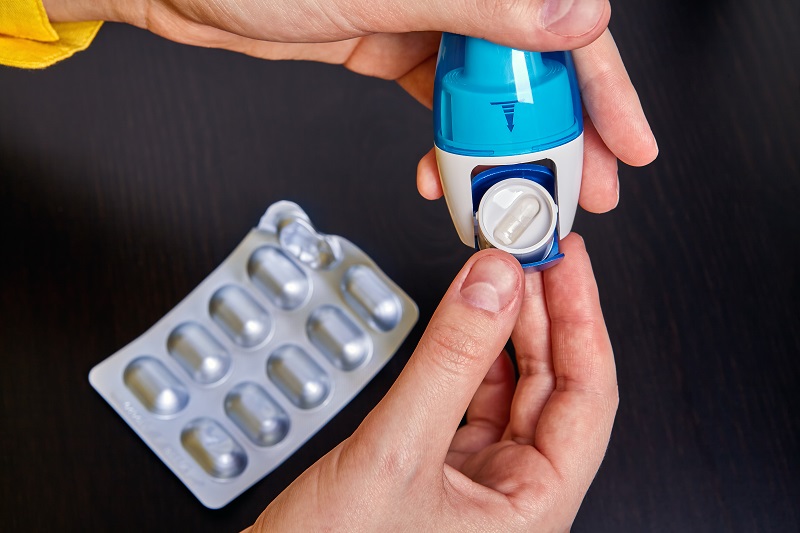
The emergence of dry powder inhalers (DPIs), particularly capsule-based systems (cDPIs), represents a significant advancement in inhalation technology. Their popularity stems from their elimination of propellants, consistent dose delivery, and superior portability.
In cDPIs, a capsule containing the medication is pierced or opened within the device, releasing the powder. The patient's inspiratory flow provides the energy to disperse the drug into fine particles, which are then inhaled through the mouthpiece for lung delivery.
DPIs make up more than 35% of the global inhalation market. Their market trajectory is notably positive, with projections indicating a compound annual growth rate of 7.2% (2021–2031) to $1.3 billion by 2031. This growth is driven by increasing acceptance among healthcare providers and patients, along with continuous technological improvements in device design and formulation.
Capsule based dry powder inhalation
The success of capsule-based DPIs heavily depends on specialised capsule design. Unlike standard oral capsules, inhalation capsules must meet stringent criteria across multiple parameters. Residual lubricant control represents a critical factor, as minimal powder retention is crucial for effective drug delivery. This necessitates precise control of lubricant quantities during manufacturing, with specialised processes developed to optimise this aspect of production.
The respiratory tract's vulnerability to infection demands stricter microbial limits compared to oral capsules. While oral delivery benefits from the protective environment of gastric acid, the respiratory system requires additional safeguards. Therefore, inhalation capsules must maintain microbial counts below 100 CFU/g, a standard significantly more stringent than that for oral capsules.
In terms of materials, these capsules are typically made from gelatin or hydroxypropyl methylcellulose (HPMC. These materials have distinct properties that can influence the performance of the final product. Globally, size 3 is the most commonly used capsule size for commercial DPI applications.
Due to their lower moisture content (3-8% w/w) and superior puncturing properties across varying humidity conditions, HPMC capsules have emerged as a preferred choice. The stability and inert nature of HPMC polymer capsules make them particularly suitable for maintaining formulation integrity and ensuring consistent aerosolisation performance.
Formulation science and engineering principles
Dry powder inhaler formulations typically combine micronised drug particles with a carrier. The carrier serves multiple purposes: it enhances powder flowability, improves dose precision, and provides sufficient bulk to achieve accurate filling weights, particularly for formulations containing small quantities of active drug.
The effectiveness of dry powder inhalation formulations relies on a complex interplay of various factors. Particle engineering represents a fundamental aspect of formulation development. Drug particles must achieve an optimal aerodynamic diameter between 1 - 5 µm for effective lung deposition. This requires sophisticated manufacturing processes and careful control of particle size distribution.
Carrier particle selection involves consideration of multiple parameters, including surface properties, particle size distribution, and compatibility with the active pharmaceutical ingredient. The drug-to-carrier ratio must be carefully balanced to ensure optimal flow properties and dose consistency. Physical interactions between particles, including cohesive and adhesive forces, play a crucial role in determining formulation performance.
Surface area considerations and static charge management become particularly important in maintaining formulation stability and ensuring consistent dose delivery. The mechanics of deagglomeration during inhalation must be understood and optimised to achieve effective drug delivery to the intended site of action.
Future directions and challenges
The approval of inhaled human insulin by the US FDA and European EMA marked a significant milestone as the first inhaled therapeutic macromolecule for systemic delivery. This success opened new possibilities in inhalation therapy, with a key advantage being its painless administration compared to injections - a factor that can improve patient compliance and treatment outcomes.
The potential of dry powder inhalation now extends beyond traditional respiratory conditions. Researchers are investigating its application in various pulmonary diseases including cystic fibrosis, lung cancer, and respiratory infections. Moreover, systemic conditions such as diabetes, Parkinson's disease, migraine, and cancer are being explored as candidates for inhalation therapy. The innovation isn't limited to new therapeutic entities - established drugs like ciprofloxacin, vancomycin, and sumatriptan are being repurposed for delivery via inhalation, expanding their therapeutic applications.
Reference
- Solanki A, Bhat J, Kalafat J, “Capsules for Dry Powder Inhalation – a Complete Product”. ONdrugDelivery, Issue 131 (Apr 2022)
- Labaki WW, Han MK, “Chronic respiratory diseases: a global view”. The Lancet, 2020, Vol 8(6), pp 531–533.
- Lavorini F, Fontana GA, Usmani OS, “New Inhaler Devices – The Good, the Bad and the Ugly”. Respiration, 2014, Vol 88(1), pp 3-15.
- Myrdal P, Sheth P, Stein SW, “Advances in Metered Dose Inhaler Technology: Formulation Development”. AAPS PharmSciTech, 2014, Vol 15(2), pp 434–455.
- Chierici V et al, “Consequences of Not-Shaking and Shake-Fire Delays on the Emitted Dose of Some Commercial Solution and Suspension Pressurized Metered Dose Inhalers”. Expert Opin Drug Del, 2020, Vol 17(7), pp 1025–1039.
- “Dry Power Inhaler Market”. Research Report, Fact.MR, Dec 2021.
- Buttini F et al, “Understanding the Importance of Capsules in Dry Powder Inhalers”. Pharmaceutics, 2021, Vol 13(11), p 1936.
- Justin K, Jnanadeva B, Fernando D, “Inhalation Delivery: Using Design of Experiments (DOE) to Optimize Inhalation Capsule’s Puncturing”. AAPS, 2017.
- Al-Tabakha MM, “HPMC Capsules: Current Status and Future Prospects”. J Pharm Pharm Sci, 2010, Vol 13(3), pp 428–442.
- Hamishehkar H et al, “The Role of Carrier in Dry Powder Inhaler”. In “Recent Advances in Novel Drug Carrier Systems”, (Sezer AD, ed) 2012, pp 39–66.
- Laube BL, “The Expanding Role of Aerosols in Systemic Drug Delivery, Gene Therapy, and Vaccination”. Respir Care, 2005, Vol 50(9), pp 1161–1176.

 Go Back
Go Back